Antibiotic resistance is a growing global crisis. With the rise of drug-resistant bacteria, infections that were once easily treated are now resurfacing as serious medical challenges. As concerns about antimicrobial resistance continue to rise, scientists are turning to an old alternative with new potential: phage therapy. To understand how this treatment works, we first need to look at what bacteriophages actually are.
What Are Bacteriophages?
A bacteriophage or phage for short, is a virus that infects and replicates inside the bacteria.1 The word “bacteriophage” comes from “bacteria” and the Greek word “phagein” which means “to devour/eat.” These viruses are incredibly small, many are less than 100 nanometers in length and can't be seen with a regular microscope. Despite their size, phages are the most abundant biological entity on earth.1 They're found wherever bacteria exist, including soil, rivers, oceans, wastewater. It is estimated that there are over 1031 phages on earth.2 Each phage is highly specialized, targeting only specific types of bacteria.1
Structure of a Bacteriophage
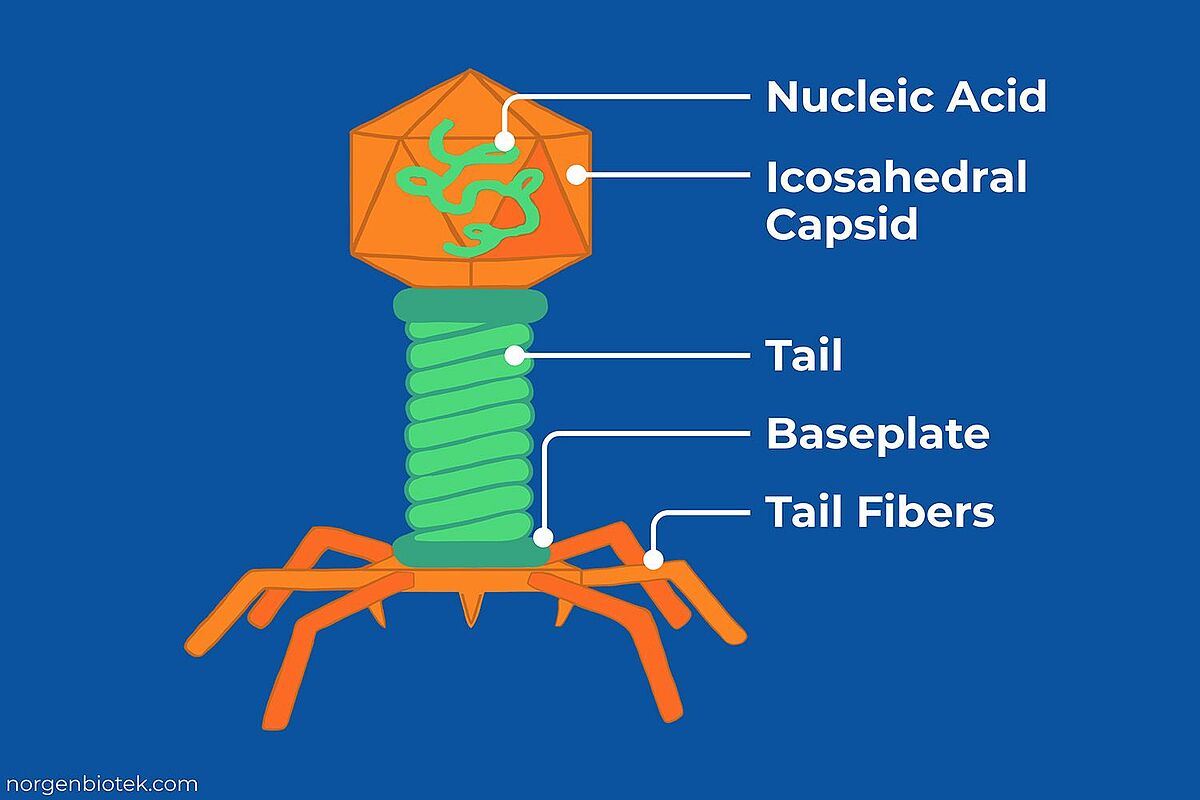
Bacteriophages display highly specialized structural organization that reflects their role in infecting bacterial hosts. Most have a protein capsid that encloses their nucleic acid genome, which can be double or single-stranded DNA or RNA. The majority of well-studied phages are tailed viruses (order Caudovirales), consisting of an icosahedral capsid connected to a tail structure. The head protects the genome and provides the high internal pressure needed for DNA injection. The tail serves as a delivery apparatus, ending in a baseplate equipped with tail fibers or spikes that recognize and bind to specific receptors on the bacterial cell structure.3 This interaction triggers conformational changes that drive genome injection into the host cytoplasm. Variations in morphology exist, including phages with short non-contractile tails, long contractile tails or filamentous forms, but all share the same fundamental purpose; recognizing a host, delivering their genome and initiating the infection cycle.4
What are Some Common Types of Bacteriophages?
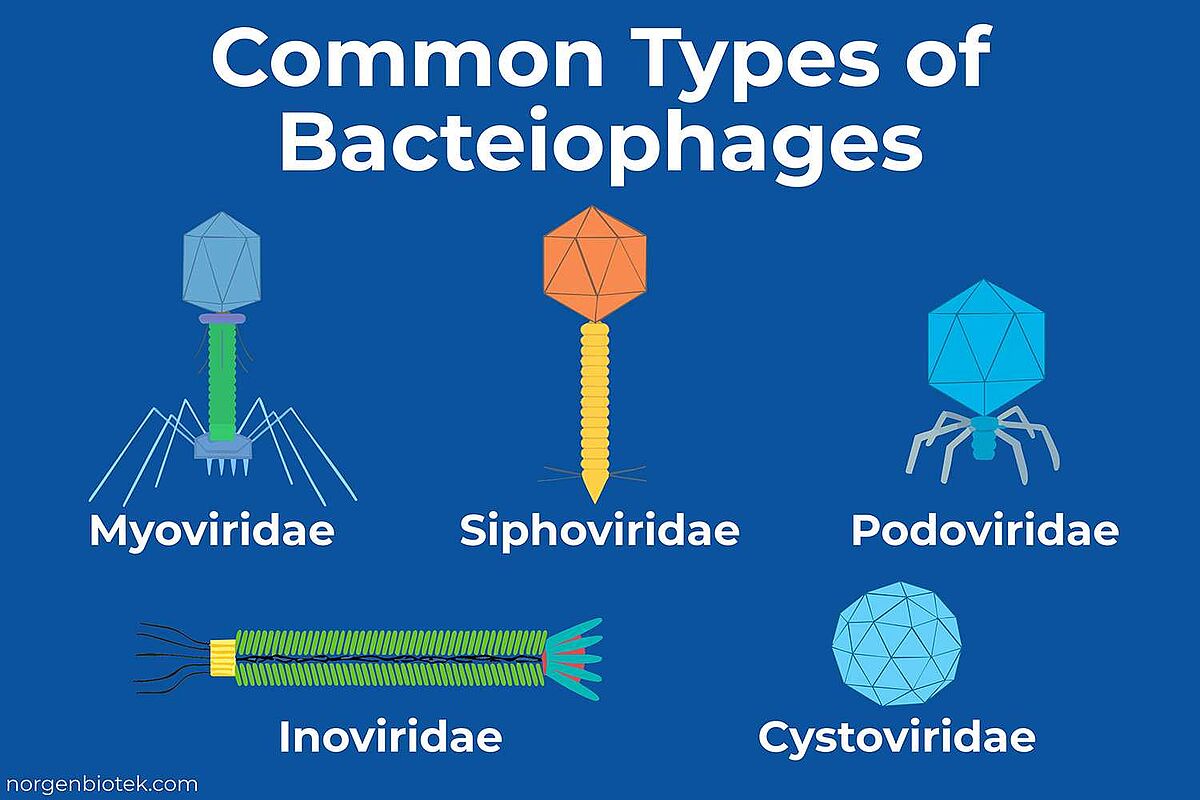
Most well-characterized bacteriophages are tailed phages historically classified under the order Caudovirales.5 These phages remain foundational in both basic research and phage therapy, and they are grouped by tail structure into three classical families:
Myoviridae
Phages in this family, such as T4, feature long, contractile tails. Upon binding to a bacterial cell, the tail sheath contracts, puncturing the cell envelope and injecting the viral genome.6 Many myoviruses are strictly lytic, making them strong candidates for therapeutic use.7
Siphoviridae
These phages, including lambda phage, have long, flexible, non-contractile tails. Many are temperate, meaning they can integrate into the host genome and remain dormant.8 While less common in therapy, some are being engineered for synthetic biology applications.
Podoviridae
Compact phages like T7 fall into this category. They possess short, non-contractile tails and rely on enzymatic degradation of the bacterial wall to inject their DNA.9
Other, less common morphotypes also exist:
Inoviridae (filamentous phages)
These phages are long, rod-shaped, and extrude new virions from the host without causing lysis.10
Cystoviridae
Spherical, double-stranded RNA phages like Φ6 that carry segmented genomes.11
Understanding the structural class of a phage is crucial for predicting its lifecycle, host specificity, and suitability for therapeutic or biotechnological applications.
How do Bacteriophages Kill Bacteria?
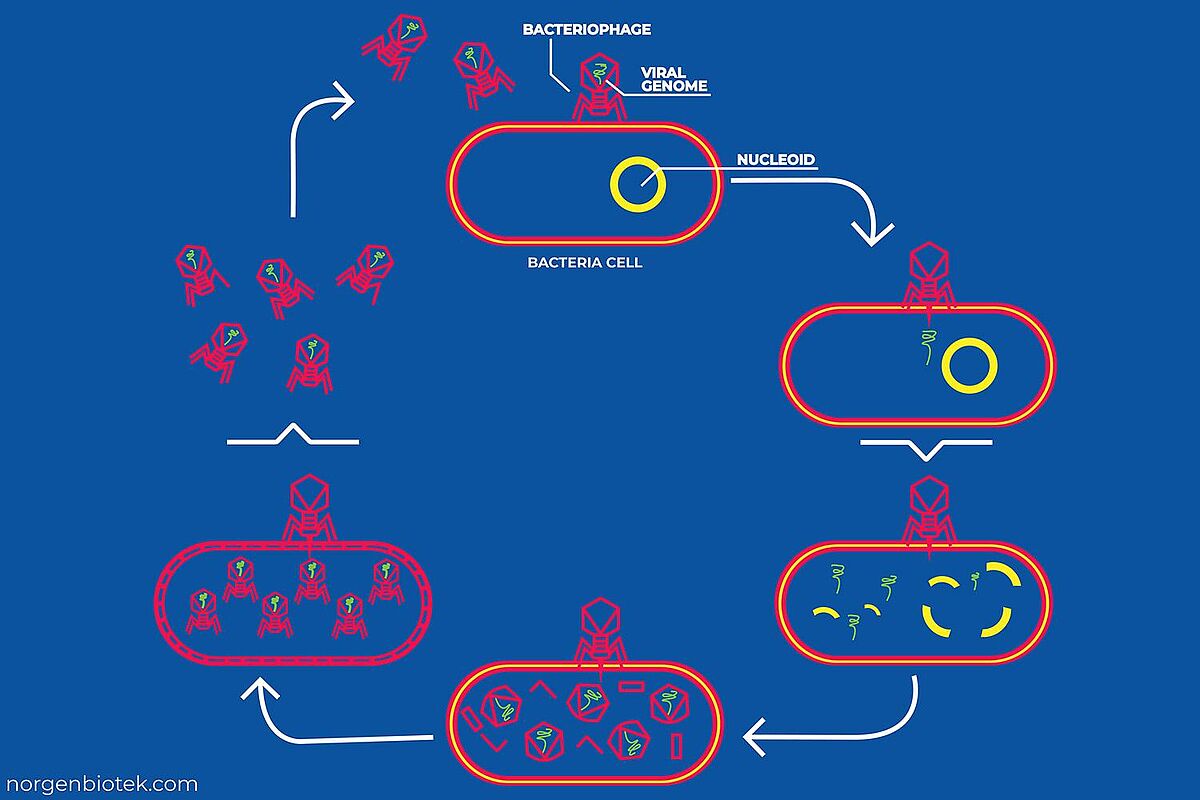
Bacteriophages are very specific, they infect only bacterial species or even particular strains within that species. After binding to a compatible host, phages initiate one of two possible replication pathways: the lytic cycle or the lysogenic cycle.1
In the lytic cycle, the phage injects its genetic material into a bacterial cell, immediately taking over the host's cellular processes. The bacterium's machinery is redirected to synthesize viral genomes and structural proteins, which then assemble into new phage particles. Once replication is complete, the host cell ruptures, either through phage encoded enzymes or as a result of accumulated pressure, releasing newly formed viruses to infect other bacteria.1
In the lysogenic cycle, the phage attaches to a susceptible bacterium and injects its genome into the host cytoplasm. Instead of replicating immediately, the phage DNA integrates into the bacterial chromosome or remains as an episome. This genetic material is then passed onto daughter cells without killing the host. When integrated, it's called a prophage, and the host is known as a lysogen. Environmental stress can trigger the prophage to reactivate, switch to the lytic cycle, and destroy the host.1
Importance of Phage DNA in Research
Evgenii Rubalskii et al. conducted a study titled “Characterization and genome analysis of the novel virulent Burkholderia phage Bm1, which is active against pan-drug-resistant Burkholderia multivorans,” focusing on a phage with therapeutic potential against a highly resistant clinical pathogen. The research team targeted Burkholderia multivorans due to its intrinsic resistance and its relevance in cystic fibrosis infections. To investigate the phage's genetic properties, the researchers used Norgen Biotek's Phage DNA Isolation Kit (Cat. 46800) to purify high-quality viral DNA suitable for both long-read sequencing with Oxford Nanopore Technologies (ONT) and high-depth Illumina sequencing. High-quality DNA extraction was critical for assembling the complete 67,539 bp genome of phage Bm1, which was analyzed to identify 113 genes, including structural, replication, and lysis-related sequences. 35 of these were functionally annotated based on sequence similarity.12
Notably, the genome lacked lysogeny-associated genes such as integrases and contained no antimicrobial resistance genes, supporting its classification as a lytic phage suitable for therapeutic use.12 This study highlights how reliable phage DNA isolation is essential for genomic characterization, a foundational step in advancing phage therapy against multidrug-resistant pathogens.
What is Phage Therapy?
A Brief History
The concept of phage therapy dates back over a century. In 1915, British bacteriologist Frederick Twort observed a mysterious agent capable of lysing bacterial cultures. Two years later, French-Canadian scientist Félix d'Hérelle independently discovered the same phenomenon and named the agent “bacteriophage”. D'Hérelle explored its therapeutic potential, using phages to treat bacterial dysentery in patients as early as 1919. By the 1920s and 30s, pharmaceutical companies in Europe and USA were producing phage-based therapeutics.13,14
Despite early excitement, inconsistent results and a limited understanding of phage biology, combined with the rapid rise of antibiotics, caused phage therapy to fall out of favour in the West by the 1940s.14 However, in the former Soviet Union, especially at the Eliava Institute in Tbilisi, Georgia, phage therapy continued to evolve.14 Today, growing concerns over antibiotic resistance have driven a resurgence in global research. Modern tools like DNA sequencing, synthetic biology, and bio-informatics are now helping scientists revisit and refine phage therapy for targeted, personalized treatment strategies.
Mechanism of Action in Phage Therapy
Phage therapy takes advantage of bacteriophages' natural ability to destroy bacteria. When a phage encounters its specific bacterial host, it binds to surface receptors and injects its DNA or RNA into the cytoplasm. This genetic takeover redirects the bacterium's machinery toward producing new viral particles, including capsid proteins and tail structures, ultimately assembling virions inside the host. Unlike broad-spectrum antibiotics, phages are highly specific, infecting only select strains without harming beneficial microbes.14
Does Phage Therapy Work?
Case Studies and Clinical Evidence
Phage therapy has a long history of use in Eastern Europe. Institutes like the Eliava Institute in Georgia and the Institute of Immunology and Experimental Therapy in Poland have been using bacteriophages for decades to treat bacterial infections like Staphylococcus aureus, E. coli, Pseudomonas, Salmonella, and Shigella. In one small case series, six patients with antibiotic-resistant diabetic foot ulcers recovered after being treated with a topical S. aureus phage. Back in 1938, a clinical trial involving 219 patients with bacterial dysentery used a multi-target phage cocktail and saw 74% improve or recover after phage treatment. During a typhoid outbreak in 1974, 18,577 children were given phages as a preventive measure, resulting in a fivefold drop in infection rates compared to placebo.14
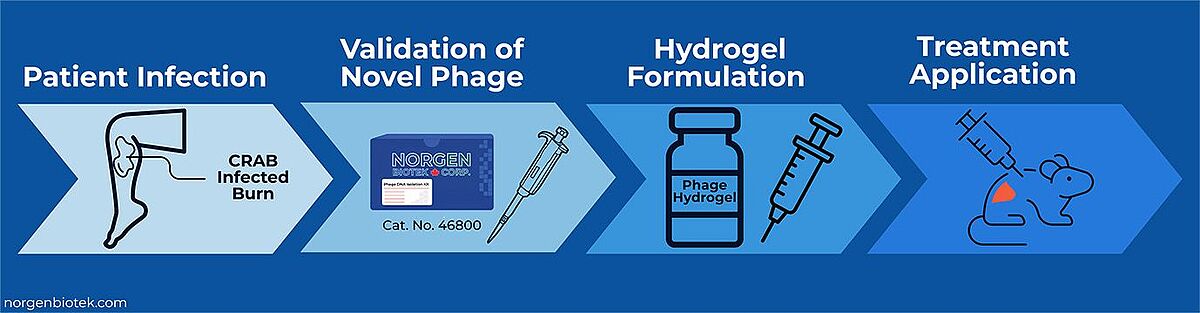
Moreover, recent studies continue to validate phage treatments. A 2025 publication reported the development of Acinetobacter baumannii phage VB_AB_Acb75, which was included into a hydrogel to treat multidrug-resistant burn wound infections. The hydrogel-phage treatment significantly accelerated wound healing and bacterial clearance in murine models. Genomic validation of the phage was performed using Norgen Biotek's Phage DNA Isolation kit (Cat. 46800), ensuring high-quality nucleic acid for downstream analysis. This study highlights both the clinical promise of phage therapy against CRAB and the importance of reliable nucleic acid extraction in therapeutic phage development.15
Phage therapy is often more effective when using cocktails, a mix of different phages targeting multiple bacterial receptors, to both broaden the bacterial range and minimize resistance emergence. A comprehensive review highlights how combination cocktails enhance the “spectrum of activity” and overcome limitations seen with single-phage treatments. Modern development strategies include matching phages to receptor diversity, genomic sequencing to ensure safety, and in vitro validation of combined effectiveness.16,17
Equally promising is phage-antibiotic synergy (PAS), which occurs when phages and antibiotics work together more effectively than either alone. While phages can disrupt biofilms and improve antibiotic penetration, this effect can vary by strain and phage type. This phenomenon has been documented in infections caused by drug-resistant pathogens where phage-induced biofilm disruption significantly improves antibiotic penetration and efficacy.18
Together, these dual strategies, carefully selected phage cocktails and phage-antibiotic combinations form a powerful, adaptive treatment paradigm. They're being actively explored in clinical settings to address multi-drug resistant, biofilm-forming bacteria for which single therapies fail.
Phage Therapy: Current Research and Applications
Antibiotic Resistant Bacteria
One of the most promising applications of phage therapy is in treating multidrug-resistant infections. Bacteriophages can selectively target resistant strains like MRSA, Pseudomonas aeruginosa, and Acinetobacter baumannii, which are increasingly unresponsive to antibiotics. All these are listed as critical threats by the WHO.19 Phages can precisely target these pathogens without harming the broader microbiome.
One notable case by Dr. Robert Schooley's team at UC San Diego used a customized phage cocktail to successfully treat a life-threatening A. baumannii infection after antibiotics failed. In collaboration with the U.S. Navy Medical Research Center and other academic partners, Schooley's team developed a personalized phage cocktail tailored to the patient's bacterial isolate. The phages were administered intravenously and percutaneously, remarkably, within days of treatment, the patient began to recover, showing reductions in bacterial loads, improved organ function, and eventual discharge from the ICU. This case not only highlighted the life-saving potential of phages in combating antibiotic-resistant infections but also emphasized the need for rapid access to sequencing, phage matching, and regulatory coordination.20
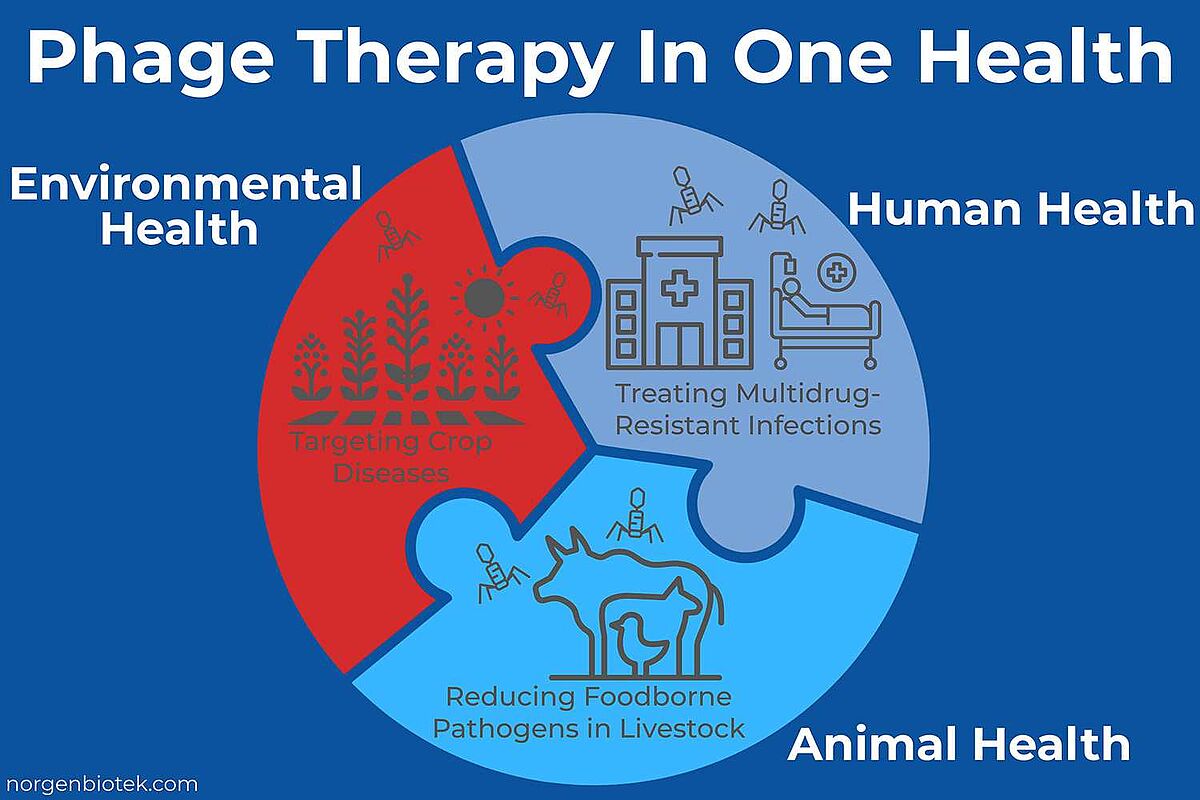
Phage Therapy in Agriculture
Phage therapy is gaining traction in agriculture as a sustainable alternative to chemical pesticides and antibiotics. Bacteriophages are used to target specific plant pathogens like Xanthomonas, Erwinia, Pseudomonas species that cause major crop diseases.21 Commercial products such as AgriPhage are already approved by the U.S. EPA and used on tomatoes, peppers, and apples.21 Unlike broad-spectrum chemicals, phages are highly specific and leave beneficial microbes unharmed. In livestock, phages are being tested to reduce gut colonization of Salmonella, E. coli, and Clostridium without contributing to antimicrobial resistance.22 Challenges remain in delivery, environmental stability, and regulatory consistency. Still, phage-based solutions hold promise for integrated pest management and One Health-focused farming practices.
Phage Therapy for the Environment
Phage therapy is emerging as a targeted strategy for managing bacterial contamination in water systems, waste systems and aquaculture. Bacteriophages can be applied to selectively reduce harmful bacteria like Vibrio, Aeromonas, and Pseudomonas in fish farms, helping to prevent disease outbreaks without distributing surrounding ecosystems.23 In wastewater treatment, phages are being investigated to combat antibiotic-resistant bacteria and limit their spread into natural water bodies.24 Their specificity offers an eco-friendly alternative to chemical disinfectants, with minimal impact on beneficial microbes. However, environmental challenges such as UV degradation, fluctuating pH, temperature shifts, salinity changes, and high organic matter content can limit phage activity.25 Despite this, phage-based biocontrol is gaining interest as a sustainable tool for environmental microbiome management and AMR reduction efforts.
Innovations in Phage Therapy Research
Phage therapy is moving beyond experimental use and becoming part of real-world clinical, environmental, and agricultural strategies. Researchers are building phage libraries that can be matched to pathogens, exploring phage-antibiotics combinations to overcome resistance, and developing delivery formats that improve targeting and stability. Engineered phages now include features like CRISPR-based editing or biofilm-degrading enzymes.26 In parallel, advances in genome sequencing and host-range prediction tools are speeding up phage selection and characterization.
Norgen Biotek's Role in Supporting Phage Research
Shengyi Han et al. (2025) conducted a study titled “Genetic characterization of four bacteriophages infecting Salmonella from fecal sewage samples,” aiming to evaluate phages as potential tools for controlling Salmonella. The researchers chose fecal sewage from multiple farms to reflect real-world reservoirs and transmission routes, key considerations in environmental and agricultural biosecurity. The study focused on phages capable of infecting Salmonella, a food-borne pathogen relevant to both livestock and human health.
Fecal Sewage samples were collected from multiple farms and used to isolate phages that infect Salmonella strains. The team extracted viral DNA using Norgen Biotek's Phage DNA Isolation Kit (Cat. 46800), chosen for its ability to efficiently purify viral nucleic acids from complex, debris-rich samples like sewage. The protocol eliminated the need for phenol-chloroform or ultracentrifugation, allowing the researchers to move directly into whole genome sequencing (WGS).
The genomes of four distinct phages were successfully assembled and annotated. Key features such as integrases, and lysis modules were characterized to assess the phages' therapeutic and biocontrol potential.27 This study underscores how reliable nucleic acid extraction, even from debris-rich environmental samples, is foundational to evaluating wild phages for biosecurity applications.
Why Phage Therapy is the Future of Medicine
Antibiotic resistance is outpacing drug development. More bacteria are becoming untreatable with existing antibiotics, and few new options are in the pipeline. Phages offer an alternative that works differently: they infect and kill specific bacterial strains, they can evolve in response to resistance, and they don't disrupt the microbiome. This makes them a natural fit for personalized medicine, especially in hard-to-treat infections like Pseudomonas, Acinetobacter or Mycobacterium abscessus.
Phage therapy also opens doors that antibiotics can't. It's being explored for targeting biofilms on medical devices, treating chronic wounds, and even preventing infections in transplant patients. Outside the clinic, phages are already in use in agriculture and aquaculture, supporting One Health strategies to reduce antimicrobial resistance across ecosystems. As more case studies, regulatory precedents, and GMP-compatible manufacturing platforms become available, phage therapy is shifting from “what if” to “what's next.”
Conclusion: The Potential of Phage Therapy
Phage therapy is no longer theoretical, it's becoming a practical solution to some of the toughest challenges in modern medicine and microbiology. With their ability to selectively target bacteria, bypass traditional resistance mechanisms, and integrate into precision medicine frameworks, bacteriophages represent a new class of biologics. Clinical trials, environmental surveillance, and regulatory pilot programs are laying the groundwork for broader adoption. Just as importantly, new tools for phage isolation, DNA purification, and genomic characterization including technologies like Norgen Biotek's phage workflow, are accelerating how quickly phages can be validated and deployed. From the ICU to agriculture, and from wastewater to personalised infection care, phages are proving to be powerful and flexible addition to the antimicrobial arsenal. Their full potential is still unfolding, but the foundation is already here.
This article was originally posted on the Norgen Biotek Blog.
Frequently Asked Questions (FAQs) About Phage Therapy
Is Phage Therapy FDA Approved?
Not as a standard, broad-spectrum medical treatment in the U.S. Phage therapy can be used under expanded access or compassionate use protocols, and some phage-derived enzymes (like endolysins) have regulatory approval. In countries like Georgia and Poland, phage therapy is part of routine clinical care.
Can Bacteria Become Resistant to Phages?
Yes, resistance can develop, often through receptor mutations, but it can be managed by combining multiple phages in therapy or by sourcing new phages. This adaptability is one of the advantages of phage-based treatment compared to fixed-spectrum antibiotics.
Are Bacteriophages Viruses?
Yes, bacteriophages are viruses that specifically infect and replicate within bacteria. Like other viruses, they rely on a host cell to reproduce and are highly specific to the bacterial species they target.
How can Norgen Biotek Products Assist in Phage Therapy Research?
Norgen's Phage DNA Isolation Kit provides researchers with the high-purity DNA needed for precise analysis and therapy design.
References
- Kasman, L. M.; Porter, L. D. Bacteriophages. StatPearls; StatPearls Publishing: Treasure Island (FL). 2025.
- Keen, E. C. A Century of Phage Research: Bacteriophages and the Shaping of Modern Biology. BioEssays News Rev. Mol. Cell. Dev. Biol. 2015, 37 (1), 6-9. https://doi.org/10.1002/bies.201400152.
- Taslem Mourosi, J.; Awe, A.; Guo, W.; Batra, H.; Ganesh, H.; Wu, X.; Zhu, J. Understanding Bacteriophage Tail Fiber Interaction with Host Surface Receptor: The Key “Blueprint” for Reprogramming Phage Host Range. Int. J. Mol. Sci. 2022, 23 (20), 12146 https://doi.org/10.3390/ijms232012146.
- Naureen, Z.; Dautaj, A.; Anpilogov, K.; Camilleri, G.; Dhuli, K.; Tanzi, B.; Maltese, P. E.; Cristofoli, F.; De Antoni, L.; Beccari, T.; Dundar, M.; Bertelli, M. Bacteriophages Presence in Nature and Their Role in the Natural Selection of Bacterial Populations. Acta Bio Medica Atenei Parm. 2020, 91 (Suppl 13), e2020024. https://doi.org/10.23750/abm.v91i13-S.10819.
- Sanz-Gaitero, M.; Seoane-Blanco, M.; van Raaij, M. J. Structure and Function of Bacteriophages. Bacteriophages; Springer, Cham, 2021, pp 19-91. https://doi.org/10.1007/978-3-319-41986-2_1.
- Maghsoodi, A.; Chatterjee, A.; Andricioaei, I.; Perkins, N. C. How the Phage T4 Injection Machinery Works Including Energetics, Forces, and Dynamic Pathway. Proc. Natl. Acad. Sci. U. S. A. 2019, 116 (50), 25097-25105. https://doi.org/10.1073/pnas.1909298116.
- Myoviridae - an overview | ScienceDirect Topics. https://www.sciencedirect.com/topics/immunology-and-microbiology/myoviridae (accessed 2025-07-29).
- Siphoviridae - an overview | ScienceDirect Topics. https://www.sciencedirect.com/topics/medicine-and-dentistry/siphoviridae (accessed 2025-07-29).
- Podoviridae - an overview | ScienceDirect Topics. https://www.sciencedirect.com/topics/immunology-and-microbiology/podoviridae (accessed 2025-07-29).
- Hay, I. D.; Lithgow, T. Filamentous Phages: Masters of a Microbial Sharing Economy. EMBO Rep. 2019, 20 (6), e47427. https://doi.org/10.15252/embr.201847427.
- Sun, Y.; Qiao, X.; Mindich, L. Construction of Carrier State Viruses with Partial Genomes of the Segmented dsRNA Bacteriophages. Virology. 2004, 319 (2), 274-279. https://doi.org/10.1016/j.virol.2003.10.022.
- Rubalskii, E.; Sedlacek, L.; Hegermann, J.; Knegendorf, L.; Salmoukas, C.; Mueller, C.; Schwerk, N.; Schlüter, D.; Ruhparwar, A.; Kuehn, C.; Ruemke, S. Characterization and Genome Analysis of the Novel Virulent Burkholderia Phage Bm1, Which Is Active against Pan-Drug-Resistant Burkholderia Multivorans. Arch. Virol.. 2025, 170 (5), 106. https://doi.org/10.1007/s00705-025-06282-w.
- Aswani, V. H.; Shukla, S. K. An Early History of Phage Therapy in the United States: Is It Time to Reconsider? Clin. Med. Res. 2021, 19 (2), 82-89 https://doi.org/10.3121/cmr.2021.1605.
- Lin, D. M.; Koskella, B.; Lin, H. C. Phage Therapy: An Alternative to Antibiotics in the Age of Multi-Drug Resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8 (3), 162-173. https://doi.org/10.4292/wjgpt.v8.i3.162.
- Sherif, M. M.; Abdelaziz, N. A.; Alshahrani, M. Y.; Saleh, S. E.; Aboshanab, K. M. In Vitro, Genomic Characterization and Pre-Clinical Evaluation of a New Thermostable Lytic Obolenskvirus Phage Formulated as a Hydrogel against Carbapenem-Resistant Acinetobacter Baumannii. Sci. Rep. 2025, 15, 17149. https://doi.org/10.1038/s41598-025-99788-x.
- Abedon, S. T.; Danis-Wlodarczyk, K. M.; Wozniak, D. J. Phage Cocktail Development for Bacteriophage Therapy: Toward Improving Spectrum of Activity Breadth and Depth. Pharmaceuticals. 2021, 14 (10), 1019. https://doi.org/10.3390/ph14101019.
- Kim, M.; Jo, Y.; Hwang, Y. J.; Hong, H. W.; Hong, S. S.; Park, K.; Myung, H. Phage-Antibiotic Synergy via Delayed Lysis. Appl. Environ. Microbiol. 2018, 84 (22), e02085-18. https://doi.org/10.1128/AEM.02085-18.
- Akturk, E.; Melo, L. D. R.; Oliveira, H.; Crabbé, A.; Coenye, T.; Azeredo, J. Combining Phages and Antibiotic to Enhance Antibiofilm Efficacy against an in Vitro Dual Species Wound Biofilm. Biofilm. 2023, 6, 100147. https://doi.org/10.1016/j.bioflm.2023.100147.
- WHO bacterial priority pathogens list, 2024: Bacterial pathogens of public health importance to guide research, development and strategies to prevent and control antimicrobial resistance. https://www.who.int/publications/i/item/9789240093461 (accessed 2025-08-05).
- Schooley, R. T.; Biswas, B.; Gill, J. J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J. J.; Reed, S. L.; Rohwer, F.; Benler, S.; Segall, A. M.; Taplitz, R.; Smith, D. M.; Kerr, K.; Kumaraswamy, M.; Nizet, V.; Lin, L.; McCauley, M. D.; Strathdee, S. A.; Benson, C. A.; Pope, R. K.; Leroux, B. M.; Picel, A. C.; Mateczun, A. J.; Cilwa, K. E.; Regeimbal, J. M.; Estrella, L. A.; Wolfe, D. M.; Henry, M. S.; Quinones, J.; Salka, S.; Bishop-Lilly, K. A.; Young, R.; Hamilton, T. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter Baumannii Infection. Antimicrob. Agents Chemother. 2017, 61 (10), e00954-17. https://doi.org/10.1128/AAC.00954-17.
- Vu, N. T.; Oh, C.-S. Bacteriophage Usage for Bacterial Disease Management and Diagnosis in Plants. Plant Pathol. J. 2020, 36 (3), 204-217. https://doi.org/10.5423/PPJ.RW.04.2020.0074.
- Gildea, L.; Ayariga, J. A.; Robertson, B. K. Bacteriophages as Biocontrol Agents in Livestock Food Production. Microorganisms. 2022, 10 (11), 2126. https://doi.org/10.3390/microorganisms10112126.
- Liu, R.; Han, G.; Li, Z.; Cun, S.; Hao, B.; Zhang, J.; Liu, X. Bacteriophage Therapy in Aquaculture: Current Status and Future Challenges. Folia Microbiol. (Praha). 2022, 67 (4), 573-590. https://doi.org/10.1007/s12223-022-00965-6.
- Withey, S.; Cartmell, E.; Avery, L. M.; Stephenson, T. Bacteriophages—Potential for Application in Wastewater Treatment Processes. Sci. Total Environ. 2005, 339 (1), 1-18. https://doi.org/10.1016/j.scitotenv.2004.09.021.
- Choudhary, M.; Bankole, I. A.; McDuffee, S. T.; Parajuli, A.; Poudel, M.; Balogh, B.; Paret, M. L.; Jones, J. B. Bacteriophages as Agents for Plant Disease Control: Where Are We After a Century? Viruses. 2025, 17 (8), 1033. https://doi.org/10.3390/v17081033.
- Łobocka, M.; Dąbrowska, K.; Górski, A. Engineered Bacteriophage Therapeutics: Rationale, Challenges and Future. Biodrugs. 2021, 35 (3), 255-280. https://doi.org/10.1007/s40259-021-00480-z.
- Han, S.; Li, S.; Li, L.; Li, S. Genetic Characterization of Four Bacteriophages of Salmonella Enterica Derived from Different Geographic Regions in China via Genomic Comparison. Res. Vet. Sci. 2025, 189, 105608. https://doi.org/10.1016/j.rvsc.2025.105608..
Featured supplier

Norgen Biotek
Norgen Biotek are experts in sample collection and preparation. In addition to the topselling RNA Purifiation Kit line, Norgen also offers tools for the collection and analysis of microbiome samples.