GMP cytokines and growth factors by Proteintech
Proteintech has completed the ISO 13485 certification for medical devices, allowing them to offer their high quality HumanKine® human cell-expressed cytokines and growth factors in GMP-compliant versions. HumanKine® GMP recombinant proteins offer safety, purity, stability, and potency to use in ex vivo manufacturing processes.

Cytokines and Growth Factors
Human cell (HEK293) expressed cytokines and growth factors with high bioactivity, stability, lot-to-lot consistency, and native human conformation & post-translational modifications.
Our human expression system ensures that proteins have native conformation and post-translational modifications to optimize biological activity.
- Animal component free
- Endotoxin free
- Xeno free
- Tag free
- Carrier free

HumanKine® recombinant proteins
Proteins expressed in E. coli do not possess post- translational modifications, such as phosphorylation or glycosylation. However, many proteins require post-translational modifications for optimal activity. HumanKine® recombinant proteins are expressed in HEK293 cells and therefore display all naturally ocurring post-translational modifications.
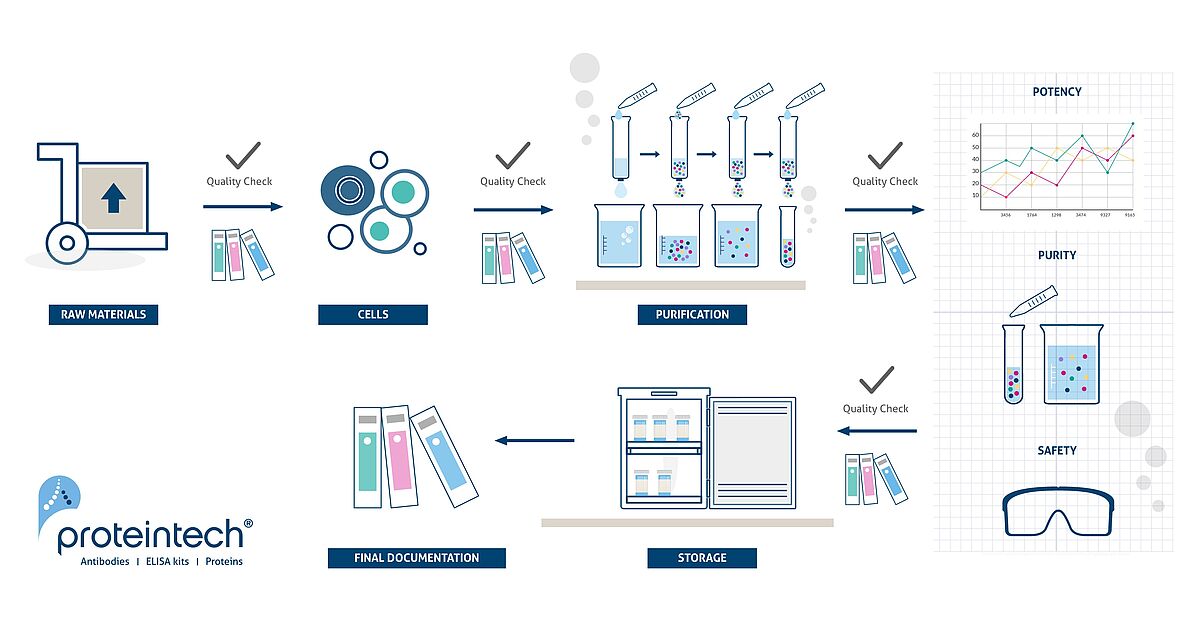
All Humankine® recombinant proteins are produced in Proteintech's in-house cGMP grade laboratory in an animal-free environment and adhering to strict quality control regulations.
- Authentic human proteins
- Native human conformation & post-translational modifications
- High bioactivity & stability
- Animal, tag and xeno-free
- Seamless transition from research to cGMP
Research Grade vs cGMP Grade
In order to facilitate a smooth transition from preclinical to clinical use, Humankine® offers a wide range of Research Use Only (RUO) and cGMP grade recombinant proteins which are exclusively expressed and purified from HEK293 human cells. The RUO and cGMP products are both manufactured using the same expression systems, production process and operating procedures in a cGMP facility. This guarantees that there is no variability in the quality and performance of these products.
The key differentiator of our cGMP products from RUO is that we adhere to a rigorous quality process that is ISO13485 certified and cGMP compliant. The cGMP grade products come with extensive documentation, thorough QC testing and traceability. The RUO grade offers reliable products which are more cost-effective during early research and development.
cGMP Grade Cytokines & Growth Factors
Use cGMP products for manufacturing of investigational products, and for manufacturing commercial therapeutic products. In vitro recombinant protein production can be prone to variability due to various reasons ranging from quality of raw materials to inconsistency in the process. HumanKine® cGMP recombinant proteins are manufactured and validated in accordance with our ISO 13485 quality management system and is compliant with cGMP.
HumanKine® cGMP recombinant proteins offer safety, purity, stability, and potency to use in ex vivo manufacturing processes, as defined in USP (United States Pharmacopeia) Chapter 1043 Ancillary Materials for Cell, Gene, and Tissue-Engineered Products, Growth Factors and Cytokines Used in Cell Therapy Manufacturing, WHO TRS, No. 822, 1992 Annex 1 Good Manufacturing Practices for Biological Products.
Proteintech is ISO13485 certified and our cGMP Policy ensures:
- Raw material qualification, documentation and traceability
- Regular check and qualification of clean rooms and instruments
- Validated process and standard operating procedures
- Well defined standards and In Process Quality Check (IPQC)
- Systematic and organized training program for personnel
- Deviations and Corrective Action Preventive Action (CAPA)
Research Grade Cytokines and Growth Factors
To facilitate a seamless transition from pre-clinical to clinical studies, both Research Grade and GMP Grade HumanKine cytokines and growth factors are manufactured by the same production process, minimizing variability in performance between Research Grade and GMP Grade products.
- cGMP Grade available
- HEK293 human cell expressed
- Authentic human proteins
- Native human folding & glycosylation
- Animal component free
- Tag, xeno and endotoxin free
- High purity
- High activity
- Manufactured in the USA
If you are interested in GMP products (even if not listed), please contact us for more information. Proteintech estimates ca. 8 weeks production time for the GMP production of any of their regular HEK293 produced products.
Further information
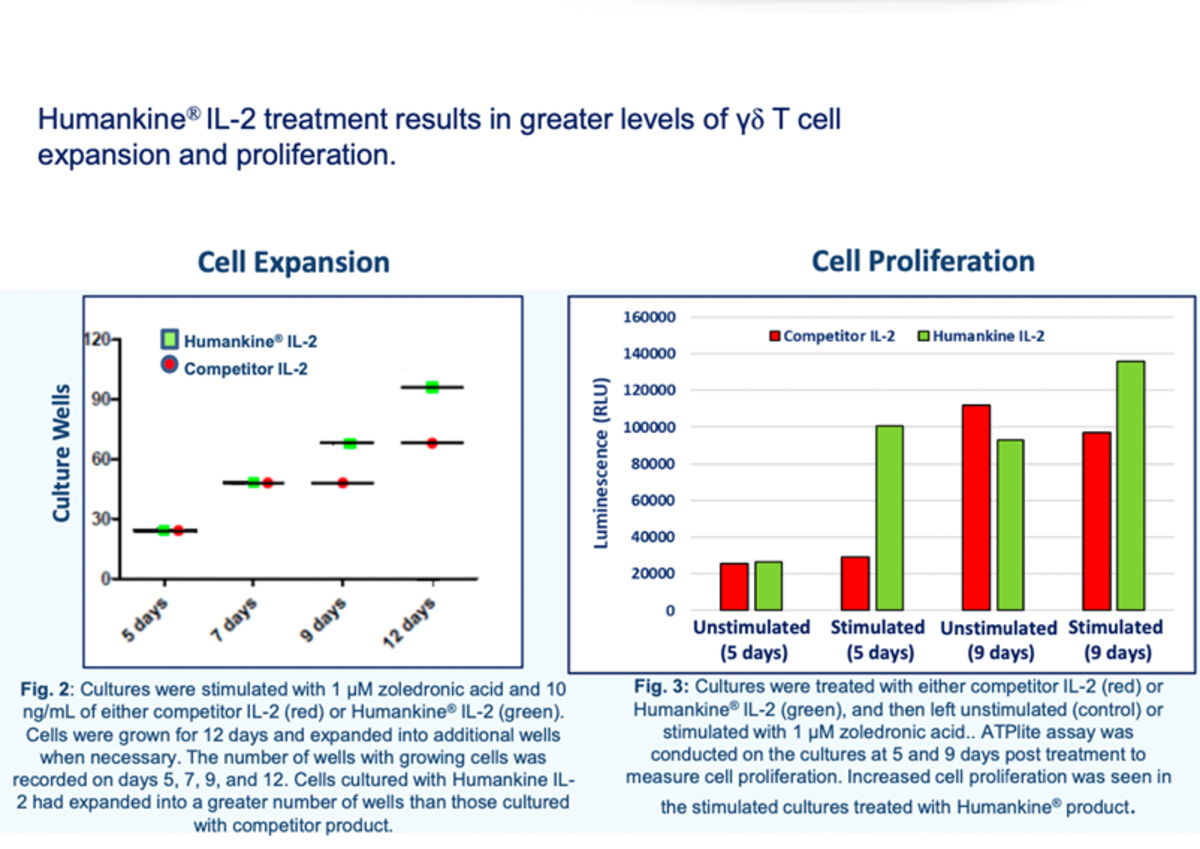
Have you ever wondered, why sometimes cell proliferation or differentiation is not great. The reason could be the source of recombinant proteins. This internal study at Proteintech demonstrates the efficiency of T cell proliferation using Human cell expressed vs bacterially expressed Interleukin-2. It shows Human cell expressed recombinant proteins has definite advantages in cell culture applications.
Top 4 considerations when choosing your recombinant protein
Due to the importance in cell culture and therapeutic systems, there are several considerations when choosing a recombinant protein for your research.
GMP grade cytokines and growth factors
Cytokines and growth factors are small, secreted proteins or peptides. Learn more about their key role in signaling and disease.