Oligomeric Alpha Synuclein, Tau, and Amyloid Beta in Neurodegenerative Diseases
The conversion of protein monomers into soluble oligomers and ultimately more complex fibrillar structures is common to virtually all neurodegenerative diseases. However, while the higher-order proteins were once considered the main species responsible for neurodegeneration, the oligomeric intermediates are now thought to be more toxic.1-3 As a result, protein oligomers have become essential tools for studying neurodegenerative pathology. StressMarq Biosciences is a leading provider of oligomeric protein constructs for neurodegenerative disease research, as evidenced by product citations in many peer-reviewed scientific journals. This article explores the role of protein oligomers in neurodegenerative disease and explains how StressMarq’s products can be used for disease modeling in drug discovery and pre-clinical investigation.
The central role of oligomers in neurodegeneration
Neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) are characterized by cognitive decline, impairment of motor skills, and reduced functional capabilities, which result from the progressive loss of neurons within the brain. While the precise causes of neurodegeneration have yet to be determined, abnormal protein aggregation is believed to be a major contributing factor. The oligomers formed from monomeric alpha synuclein (α-synuclein), tau, and amyloid beta (Aβ) are of particular interest to researchers since they have been linked to some of the most common neurodegenerative conditions.
Alpha synuclein oligomers
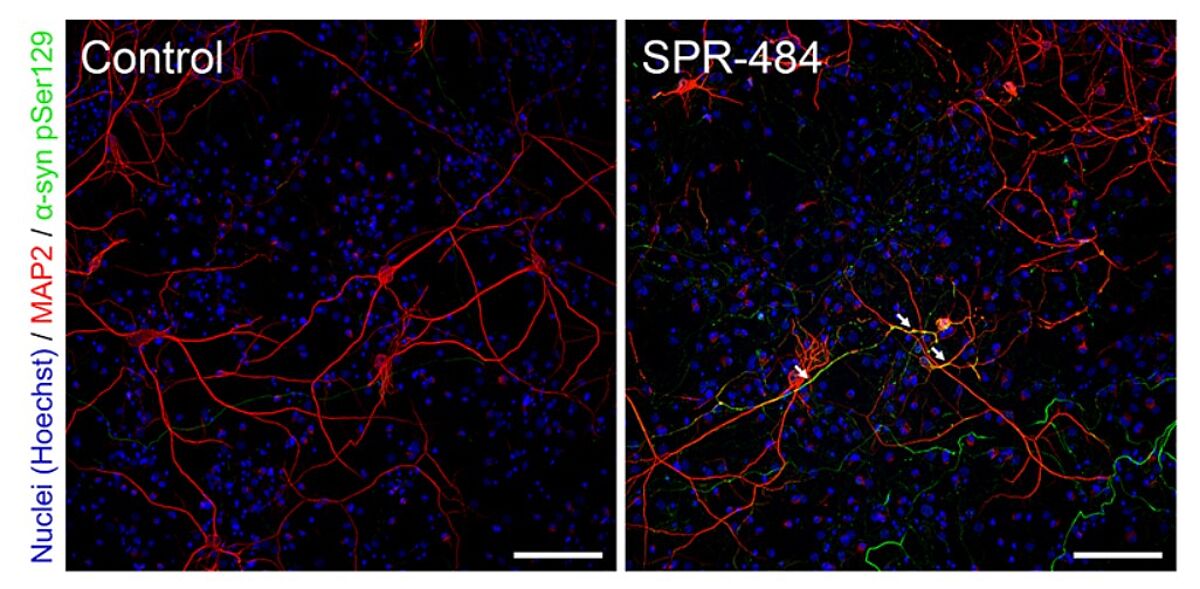
Alpha synuclein, encoded by the SNCA gene, is a 14 kDa protein that is expressed ubiquitously in neurons. Although its physiological function is unclear, suggested roles include regulation of the SNARE complex, which controls neurotransmitter release in the pre-synapse. Alpha synuclein was first associated with PD in 1997, when a point mutation (A53T) in SNCA was correlated with familial disease. It is now known that PD pathogenesis involves the aggregation of α-synuclein monomers into soluble oligomers that can spread throughout the brain. Here, they act as seeds for further monomers to oligomerize and may also form larger insoluble intracellular fibrils with a predominantly beta sheet structure, such as those found in Lewy bodies.
Tau oligomers
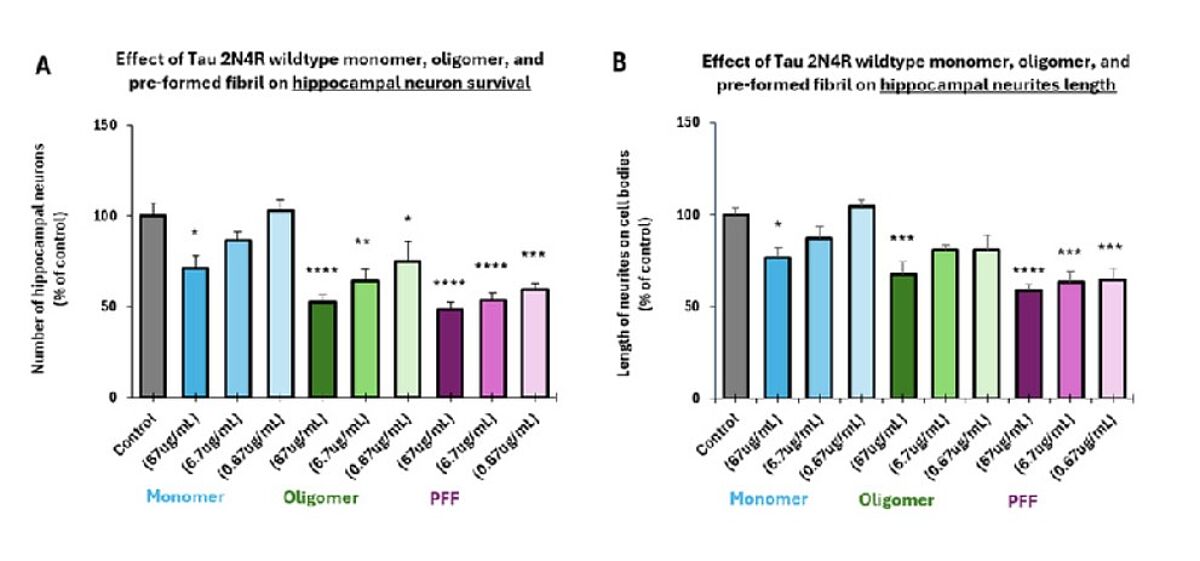
Tau, encoded by the MAPT gene and expressed as six different isoforms due to alternative splicing, is a microtubule-associated protein that serves to stabilize the neuronal cytoskeleton and facilitate the axonal transport of nutrients and other molecules. During the development of AD and other so-called tauopathies, tau becomes hyperphosphorylated and loses its affinity for microtubules, resulting in the formation of soluble tau oligomers. These diffuse throughout the brain, exerting their toxic effects through mechanisms that include destabilizing microtubules, disrupting synaptic function, and triggering chronic inflammation. Additionally, tau oligomers can give rise to insoluble intracellular neurofibrillary tangles (NFTs), one of the key pathological hallmarks of AD.
Amyloid beta oligomers
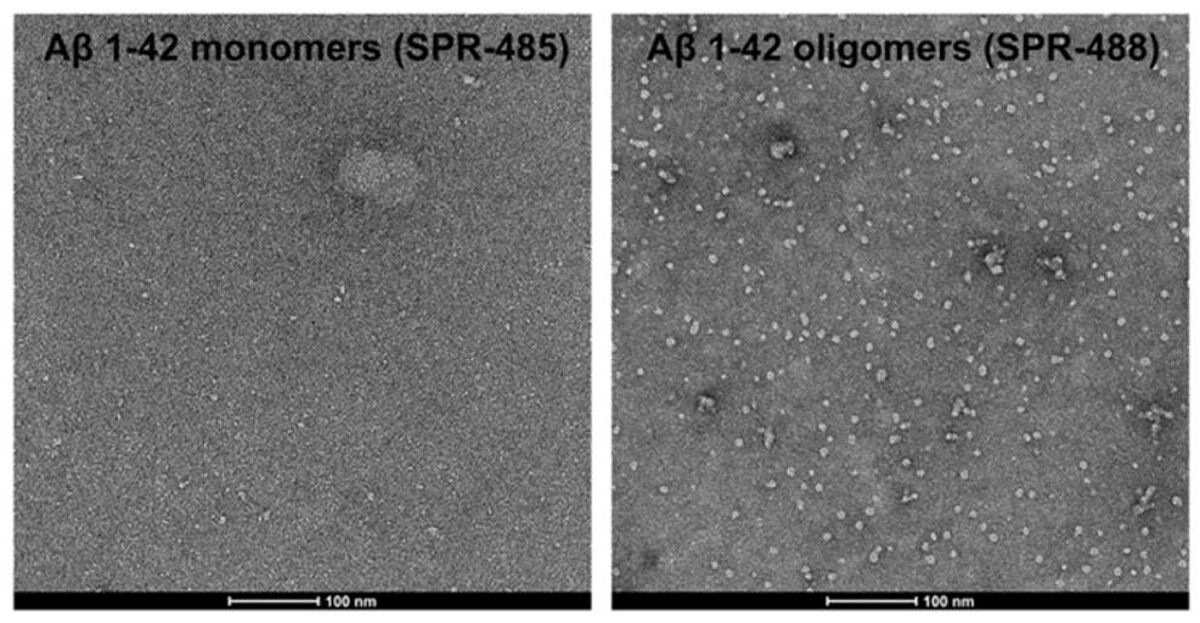
Amyloid beta is a 4 kDa peptide derived from cleavage of the amyloid precursor protein (APP), a transmembrane protein that is widely expressed and has roles including synapse formation, neural plasticity, antimicrobial activity, and mineral transport. Under normal conditions, Aβ peptides are cleared from the brain via mechanisms that include internalization and degradation by microglia. However, in AD, Aβ peptides accumulate and can assemble into oligomers. These impair neuronal function through binding to synaptic receptors, which leads to their degradation, and disrupting signaling by key neurotransmitters, including glutamate and acetylcholine. Aβ oligomers may also trigger inflammatory immune responses and perturb cellular homeostasis, as well as give rise to the extracellular amyloid plaques that characterize AD brain pathology.
Oligomers as targets for drug development
Mounting evidence suggests that α-synuclein, tau, and Aβ oligomers may be more relevant targets for drug development than the aggregates they give rise to, owing to their enhanced toxicity. For example, a comparison of small α-synuclein oligomers and larger fibrillar species showed the oligomers to cause a greater neuroinflammatory response when added to microglia cells in vitro.4 And a study in which various tau species were incubated with neuroblastoma cells to assess cytotoxicity and seeding behavior found the tau oligomers to be more potent than the mature fibrils.5 In addition, multiple late-stage trials in AD have indicated that inhibiting Aβ oligomer toxicity represents an effective approach to slow or stop disease progression.6 Moreover, because oligomers are produced before their larger counterparts, they could potentially be used as early biomarkers for neurodegenerative disease. For instance, it has been suggested that oligomeric tau be added to the panel of cerebrospinal fluid (CSF) markers used for diagnosing AD.7
Oligomers for neurodegenerative disease research
A main challenge faced by researchers studying neurodegenerative disease lies in generating and stabilizing toxic in vitro oligomers.4 To help advance neurodegeneration research, StressMarq Biosciences has developed an extensive range of protein constructs, which includes α-synuclein, tau, and Aβ oligomers, as well as a comprehensive selection of monomers and pre-formed fibrils (PFFs).
One way in which StressMarq’s products are used involves seeding oligomers into a solution containing the corresponding monomer and a small molecule drug, then monitoring fibril formation with Thioflavin T, which emits a strong fluorescent signal upon fibril binding. Other popular methods include evaluating neuron survival and/or neurite outgrowth following the addition of oligomers to cells grown in culture, and performing live-cell imaging to track the uptake of fluorescently labeled oligomers.
To safeguard experimental accuracy and reproducibility, all of StressMarq’s neuroproteins are subject to rigorous quality control. Oligomeric structures are confirmed via size-exclusion chromatography (SEC) and AFM/TEM imaging, and all protein batches are tested for sterility and endotoxin.
This article has originally been published by Biocompare Industry Innovations.
References
1. Forloni G. Oligomers and Neurodegeneration: New Evidence. Aging Dis. 2023;14(6):1977-1980. doi:10.14336/AD.2023.0327
2. Rinauro, D.J., Chiti, F., Vendruscolo, M. et al. Misfolded protein oligomers: mechanisms of formation, cytotoxic effects, and pharmacological approaches against protein misfolding diseases. Mol Neurodegeneration 19, 20 (2024). https://doi.org/10.1186/s13024-023-00651-2
3. Zampar S, Di Gregorio SE, Grimmer G, Watts JC, Ingelsson M. "Prion-like" seeding and propagation of oligomeric protein assemblies in neurodegenerative disorders. Front Neurosci. 2024;18:1436262. Published 2024 Aug 5. doi:10.3389/fnins.2024.1436262
4. Emin D, Zhang YP, Lobanova E, et al. Small soluble α-synuclein aggregates are the toxic species in Parkinson's disease. Nat Commun. 2022;13(1):5512. doi:10.1038/s41467-022-33252-6
5. Ghag G, Bhatt N, Cantu DV, et al. Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross-seeding behavior. Protein Sci. 2018;27(11):1901-1909. doi:10.1002/pro.3499
6. Tolar M, Hey J, Power A, Abushakra S. Neurotoxic Soluble Amyloid Oligomers Drive Alzheimer's Pathogenesis and Represent a Clinically Validated Target for Slowing Disease Progression. Int J Mol Sci. 2021;22(12):6355. doi:10.3390/ijms22126355
7. Mroczko B, Groblewska M, Litman-Zawadzka A. The Role of Protein Misfolding and Tau Oligomers (TauOs) in Alzheimer's Disease (AD). Int J Mol Sci. 2019;20(19):4661. doi:10.3390/ijms20194661

StressMarq
As a market leader, StressMarq develops and commercializes a diverse range of fibrillar, oligomeric, and monomeric protein preparations for neurodegenerative disease research. These include key proteins such as alpha synuclein, tau, amyloid beta, SOD1, and TTR.