Misfolding and aggregation of the microtubule-associated protein tau into neurofibrillary tangles (NFTs) is a pathological hallmark of Alzheimer’s disease and other tauopathies. However, while NFTs have long been considered the main cause of neuronal cell death associated with these disorders, it is now thought that intermediate species of tau are more likely to blame. Understanding the pathological role of pre-fibrillar tau oligomers is critical to determine their potential for therapeutic targeting.
The tau oligomerization pathway
Tau is expressed as six different isoforms, ranging from 352 – 441 amino acids in length, which result from alternative splicing of the MAPT gene1. These are found mainly in neuronal axons, where they function to regulate microtubule assembly and stability. Each tau isoform contains a minimum of 30 distinct phosphorylation sites that, under normal conditions, are in the dephosphorylated state2. Yet, during the development of tauopathies such as Alzheimer’s disease, tau becomes hyperphosphorylated and undergoes conformational changes, leading to its dimerization and self-assembly into larger oligomeric aggregates3. These ultimately associate to form NFTs, which have been widely studied for their role in neurodegenerative disease pathology.
Figure 1: Alternative splicing of the MAPT gene and tau isoforms. Tau is encoded by the MAPT gene comprising 16 exons. Exons 0 and 1 encode the 5' untranslated sequence of MAPT mRNA while exon 14 encodes part of the 3' untranslated region. Exons 4a, 6, and 8 are only transcribed in peripheral tissues. Exons 2 and 3 encode 29-amino acid residue inserts near the amino-terminus, while exons 9-12 encode microtubule binding domain repeats near the carboxyl-terminus. Alternative splicing of MAPT results in 6 isoforms ranging from 352 to 441 amino acids. Tau isoforms are named based on the number of amino-terminal inserts (0N, 1N, and 2N) or carboxyl-terminal microtubule binding domain repeats (3R and 4R). 1
What is the neurotoxic species in tauopathies?
For many years, NFTs were considered to be the main toxic species responsible for neuronal cell death observed in tauopathies. However, more recent studies have suggested this not to be the case4. These include the finding that caspase activation (an irreversible commitment to apoptosis) precedes NFT formation in tau transgenic mice as well as the discovery that NFT-bearing neurons are able to survive despite having compromised membrane integrity5,6. Now, there is a growing body of evidence to indicate that tau oligomers are the most toxic species in disease and may also be responsible for the spread of pathology7. For example, following the sonication of tau fibrils to form tau oligomers (identifiable by Western blotting using an oligomer-specific antibody, T22) and subsequent incubation with SH‐SY5Y neuroblastoma cells, tau oligomers were found to be the toxic species and were more potent in seeding compared to mature fibrils8.
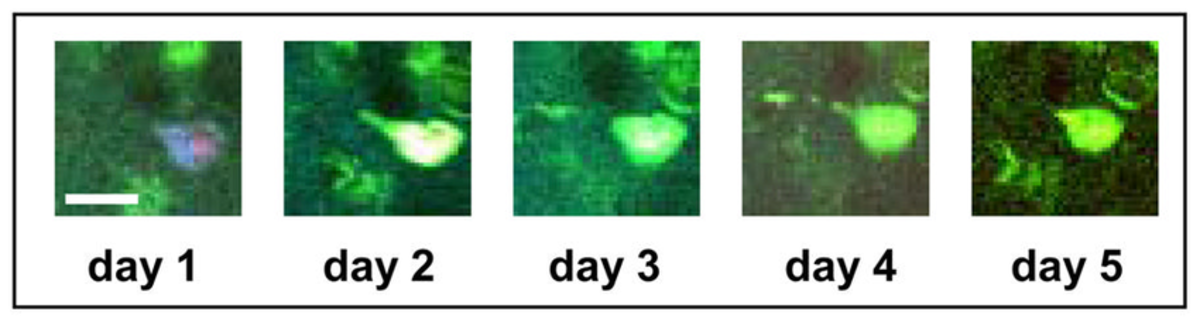
Figure 2: Tangle-bearing neurons can be imaged for several days in the living brain, and caspase activation is not associated with acute neuronal death. 5
Tau oligomers and Alzheimer’s disease
Although the pathological role of tau oligomers in Alzheimer’s disease remains unclear, it appears increasingly likely that these soluble tau species are the most deleterious form of the protein. Research has revealed tau oligomers to be characterized by the neurotoxicity, infectivity, and capacity for amplification that underlie disease progression, and it has been hypothesized that mitochondrial dysfunction caused by internalized tau oligomers may be fundamental9. Notably, elevated levels of tau oligomers may represent an important early biomarker for Alzheimer’s disease, with utility prior to the appearance of clinical symptoms10.
Targeting oligomeric forms of tau
Because tau oligomers result from hyperphosphorylation, inhibiting the kinases involved in this process is one potential approach to therapeutic intervention. Another strategy is to block tau monomers from interacting, thereby preventing oligomer formation and seeding. In the study previously described, pre-incubating tau oligomers with excess amounts of oligomer-specific T22 antibody was shown to significantly reduce cellular toxicities in vitro, thereby demonstrating the therapeutic value of targeting oligomeric forms of tau8.
Figure 3: Cell viability assay of SH‐SY5Y cells treated with 2 μM of different preparations: tau oligomers, tau fibrils and sonicated tau fibrils, and sonicated tau fibrils preincubated with T22 antibody at 1:1, 1:2, and 1:4 ratios, respectively (sonicated tau fibrils: T22 antibody). The data demonstrate that oligomers generated by sonication of preformed fibrils are highly toxic to cells. The toxicity of sonicated fibrils significantly reduced upon preincubating them with excess amount of affinity‐purified T22 antibody. Bars and error bars represent mean values and standard deviations, respectively (* P < 0.05). (** P < 0.01). 8
Reagents for the study of tau and its role in Alzheimer’s disease
StressMarq has developed a broad range of products for studying tau and its role in Alzheimer’s disease. These include high-quality tau proteins and antibodies, such as our active tau preformed fibrils (PFFs) and filaments that can be used for seeding tau aggregation and induce pathology. The scientific team at StressMarq is currently developing tau oligomers for research use.
Products
| Catalog No. | Protein | Species | Expression System | Length/Fragment | Mutant | Monomer source |
|---|---|---|---|---|---|---|
| SPR-480 | Tau 2N4R PFFs | Human | E. coli | 2N4R (441-amino acid) Full Length | Wild-type | SPR-479 |
| SPR-329 | Tau 2N4R, P301S PFFs | Human | E. coli | 2N4R (441-amino acid) Full Length | P301S | SPR-327 |
| SPR-329-A488 | Tau 2N4R, P301S PFFs (ATTO488 conjugated) | Human | E. coli | 2N4R (441-amino acid) Full Length | P301S | SPR-327 |
| SPR-471 | Tau 2N4R, P301S PFFs | Human | Baculovirus | 2N4R (441-amino acid) Full Length | P301S | SPR-473 |
| SPR-475 | Tau 2N4R, P301S PFFs | Mouse | E. coli | 2N4R (441-amino acid) Full Length | P301S | SPR-474 |
| SPR-461 | Tau dGAE PFFs | Human | E. coli | dGAE (AA297-391) Truncated | Wild-type | SPR-444 |
| SPR-462 | Tau dGAE, C322A PFFs | Human | E. coli | dGAE (AA297-391) Truncated | C322A | SPR-445 |
| SPR-330 | Tau K18, P301L PFFs | Human | E. coli | K18 (4R) Truncated | P301L | SPR-328 |
| SPR-477 | Tau K18, K280 Deletion PFFs | Human | E. coli | K18 (4R) Truncated | ΔK280 | SPR-476 |
| SPR-463 | Tau 2N4R, P301S Filaments | Human | E. coli | 2N4R (441-amino acid) Full Length | P301S | SPR-327 |
Suppliers

StressMarq
StressMarq Biosciences offers primary antibodies, antibody conjugates, proteins, immunoassay kits and small molecules.
Shop for StressMarq products

rPeptide
rPeptide offers peptides, proteins and antibodies for research on Alzheimer's and Parkinson's disease including alpha-synuclein, beta-amyloid, tau, and more.
Shop for rPeptide products

SignalChem
SignalChem has established itself as a leader in the development of innovative cell signaling products to meet the needs of scientists in basic research and drug discovery.
Shop for SignalChem products
References
- Tau‑mediated Neurodegeneration and Potential Implications in Diagnosis and Treatment of Alzheimer’s Disease, Wu XL et al, Chin Med J (Engl). 2017 Dec 20;130(24):2978-2990
- Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease, Lasagna-Reeves CA et al, FASEB J. 2012 May;26(5):1946-59
- Characterization of Prefibrillar Tau Oligomers in Vitro and in Alzheimer Disease, Patterson KR et al, J Biol Chem. 2011 Jul 1;286(26):23063-76
- Soluble forms of tau are toxic in Alzheimer’s disease, Koepeikina KJ et al. Transl Neurosci. 2012 Sep;3(3):223-233
- Caspase activation precedes and leads to tangles, de Calignon A et al, Nature. 2010 Apr 22;464(7292):1201-4
- Tangle-bearing neurons survive despite disruption of membrane integrity in a mouse model of tauopathy, de Calignon A et al, J Neuropathol Exp Neurol. 2009 Jul;68(7):757-61
- Advances in therapeutics for neurodegenerative tauopathies: moving toward the specific targeting of the most toxic tau species, Gerson JE et al, ACS Chem Neurosci. 2014 Sep 17;5(9):752-69
- Soluble tau aggregates, not large fibrils, are the toxic species that display seeding and cross‐seeding behavior, Ghag G et al, Protein Sci. 2018 Nov; 27(11): 1901–1909
- Tau Oligomers: Cytotoxicity, Propagation, and Mitochondrial Damage, Shafiei SS et al. Front Aging Neurosci. 2017 Apr 4;9:83
- The Role of Protein Misfolding and Tau Oligomers (TauOs) in Alzheimer’s Disease (AD), Mroczko B et al, Int J Mol Sci. 2019 Sep 20;20(19):4661