What are biosimilars?
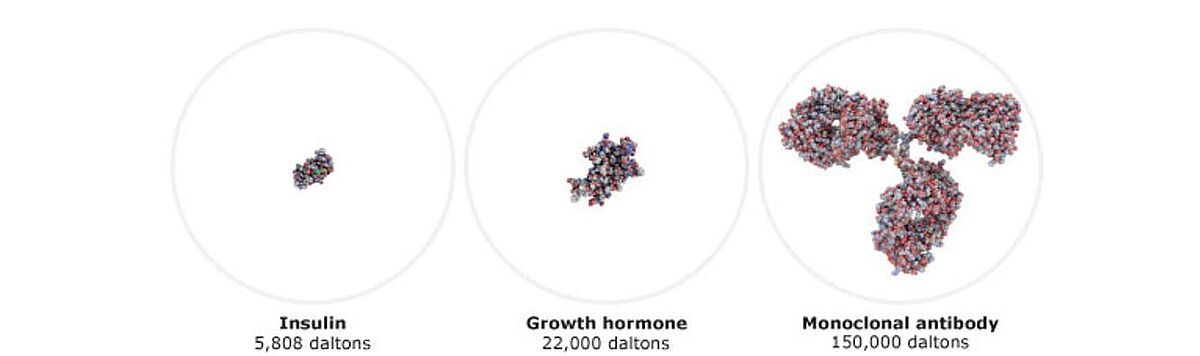
Biologics are large-molecule drugs with complex protein structures, typically produced in animal cells, yeast, or bacteria. They are approved for therapeutic use in humans, and their structure, production and formulation are tightly regulated. Examples include monoclonal antibodies used to treat multiple diseases.
The biologics market has grown substantially in recent years and is forecast to continue with an estimated compound annual growth rate of 9.1% from 2023 to 2032. One major challenge is the high cost of biologics, which limits accessibility. Biosimilars offer a solution: they are highly similar to their parent biologic drug in activity, efficacy, safety and immunogenicity¹. Their affordability makes them cost-effective alternatives for both therapeutic and research applications.
Biosimilars share the same amino acid sequence as their reference medicine, though minor differences may arise due to the complexity of protein structures. Manufacturing biosimilars requires multi-step processes, and each manufacturer uses unique cell lines and proprietary methods. As biosimilars are produced in biological systems, structural variations and batch-to-batch variability can occur².
Regulatory approval ensures any differences between a biosimilar and its reference biologic do not affect safety or effectiveness. This requires a multitude of analytical and functional assays to first characterise the reference biologic, then establish biosimilarity.
How are biosimilars different from generics?
Although often confused, biosimilars and generics are distinct. Generic drugs contain the same active ingredient as their brand-name counterparts, and both are chemically synthesised. Their simple structures make manufacturing straightforward and reproducible.
Biosimilars, in contrast, are based on biologics, which have large, three-dimensional structures. They can only be manufactured after patent and exclusivity rights expire. Their higher complexity means development takes longer and is more expensive compared with generics.

How are biosimilars developed and produced?
Developing a biosimilar begins with comprehensive characterisation of the reference molecule. Large numbers of batches are studied over time to assess potential structural variations³. Once the reference structure is defined, biosimilar production involves steps similar to the original biologic: host cell modification, cell culture, extraction, purification, refolding and formulation into a stable product.
After a highly similar candidate is produced, comparative structural and functional studies are performed. Post-translational modifications (PTMs) are closely monitored, as they can affect structure, safety and potency.
Analytical methods include:
- Mass spectrometry for peptide mapping, PTMs and glycosylation profiling.
- Spectroscopy and chromatography for higher-order structural analysis and heterogeneity.
Functional similarity is established through assays such as:
- FACS, ELISA and SPR for receptor binding.
- ADCC and CDC assays for bioactivity and apoptosis induction.
Pre-clinical and clinical testing confirm equivalent efficacy and safety, with biosimilars required to demonstrate comparable pharmacokinetics (PK) and pharmacodynamics (PD). Post-approval surveillance ensures ongoing safety. Stability testing further evaluates storage conditions, excipients and environmental stresses.
Applications of biosimilars in therapy and research
The highly regulated development of biosimilars has produced safe and effective therapeutic options now widely used in clinical practice. Their lower cost provides substantial savings for healthcare systems. The first biosimilar approved in the EU was Omnitrope (somatropin) in 2006, a recombinant growth hormone medication.
For research, biosimilars provide accessible alternatives to therapeutic biologics, which may not always be available in small quantities. Applications include:
- Proof-of-concept ligand binding assay development.
- Studying signalling pathways and cellular processes.
- Exploring disease-related mechanisms.
For example, Adalimumab biosimilar targets TNF-α and is useful for studying autoimmune disease, insulin resistance and cancer. By modulating TNF-α, researchers can investigate its role in apoptosis, inflammation, anti-tumour activity and immunoregulation.
Mouse models also benefit from biosimilars, enabling proof-of-principle studies, toxicokinetic testing, and evaluation of therapeutic activity. In one study, researchers combined a bispecific T cell engager with an Ipilimumab biosimilar (InVivoSIM anti-human CTLA-4, distributed by LubioScience) to treat colorectal cancer. The combination therapy improved survival in a peritoneal carcinomatosis mouse model⁴.
Biosimilars for antibody-drug conjugates (ADCs)
Antibody–drug conjugate (ADC) biosimilars are an emerging class of therapeutics. Kadcyla (trastuzumab emtansine) is the most commercially successful ADC, targeting HER2-positive cancers by linking an antibody to the cytotoxic drug emtansine.
The first Kadcyla biosimilar, Ujvira, was approved in India. It was shown equivalent in safety, efficacy, pharmacokinetics and immunogenicity, while significantly reducing treatment costs for HER2-positive metastatic breast cancer.
ADC biosimilars pose unique challenges due to their structural complexity. Additional analytical assays are required to measure drug-to-antibody ratios and ensure stability of the cytotoxic payload.
Conclusion
Biosimilars are now an integral part of research and therapy. Their affordability and proven safety make them valuable alternatives to biologics, driving innovation in both clinical and laboratory settings.
References
European Medicines Agency. Biosimilar medicines: overview. (2023).
Ventola, L. C. Biosimilars Part 1: Proposed regulatory criteria for FDA approval. Pharm. Ther. 38, 270–287 (2013).
Cancer Vanguard. Cancer Vanguard biosimilars presentation. Available at: https://www.youtube.com (2017).
Crupi, M. J. F. et al. Oncolytic virus driven T-cell-based combination immunotherapy platform for colorectal cancer. Front. Immunol. 13, 1065179 (2022). doi:10.3389/fimmu.2022.1065179
Suppliers

Bio X Cell
Bio X Cell is a specialist for high quality monoclonal antibodies for in vivo research. Antibodies have low endotoxin levels and no preservers.

Absolute Antibody
Absolute Antibody is an expert in engineering recombinant antibodies for in vivo research. Their custom services include hybridoma sequencing, antibody engineering and expression.

ProteoGenix
Bioreagents for therapeutic research - over 10000 recombinant antibodies, proteins, peptides, genes and custom production services.

ACROBiosystems
ACROBiosystems is a leading manufacturer of recombinant proteins and other reagents to support the development of target therapeutics, vaccines, and diagnostics.

Antibodies.com
Antibodies.com supplies high-quality biological reagents to life science researchers at cost-effective prices. They are a trusted supplier of antibodies, proteins, immunoassays, and other companion reagents. Headquartered in Cambridge (UK), they support life scientists worldwide by supplying the same high-quality products as the industry leading suppliers, that are sourced from the same primary manufacturers, but at more affordable prices.
About Antibodies.com Shop for Antibodies.com products
