Immune checkpoint inhibitors were a breakthrough both in the developmental direction as well as the clinical approval of antibody therapies for cancer immunotherapies. The specificity of antibodies provided healthcare providers with highly effective cancer therapy that minimized the unwanted side effects of chemotherapies. However, a major limitation towards its adoption as a therapy was the number of patients that were non-responsive, relapsed, or refractive towards immune checkpoint inhibitors. This brought around the exploration of alternative strategies, taking advantage of research into the immunoregulatory network from the discovery of checkpoint inhibitors. The primary alternative strategy highlighted was the direct activation of immune cells using immune agonistic antibodies (IAAs), rather than modulating the immune regulatory network. As such, IAAs are designed to enhance the immune response against cancer cells, leading to increased tumor cell cytotoxicity and immune cell survival. This article highlights a few inspiring targets of IAAs while exploring their potential in combating various cancers.
CD40 and its agonistic antibodies
CD40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is expressed on antigen-presenting cells which includes dendritic cells, B cells, and macrophages. It plays a crucial role within the immune system, enabling dendritic cells to activate antigen-specific T cells while promoting B cell activation and proliferation as well as antigen presentation.1 Antibodies targeting CD40 are designed to mimic the effects of CD40L, its natural ligand) and activate the signaling pathway that results in the maturation of dendritic cells that subsequently activate and expand antigen-specific T cells. Several current therapeutic antibodies targeting CD40 include APX005M, ChiLob7/4, ADC_1013, SEA-CD40, and selicrelumab.
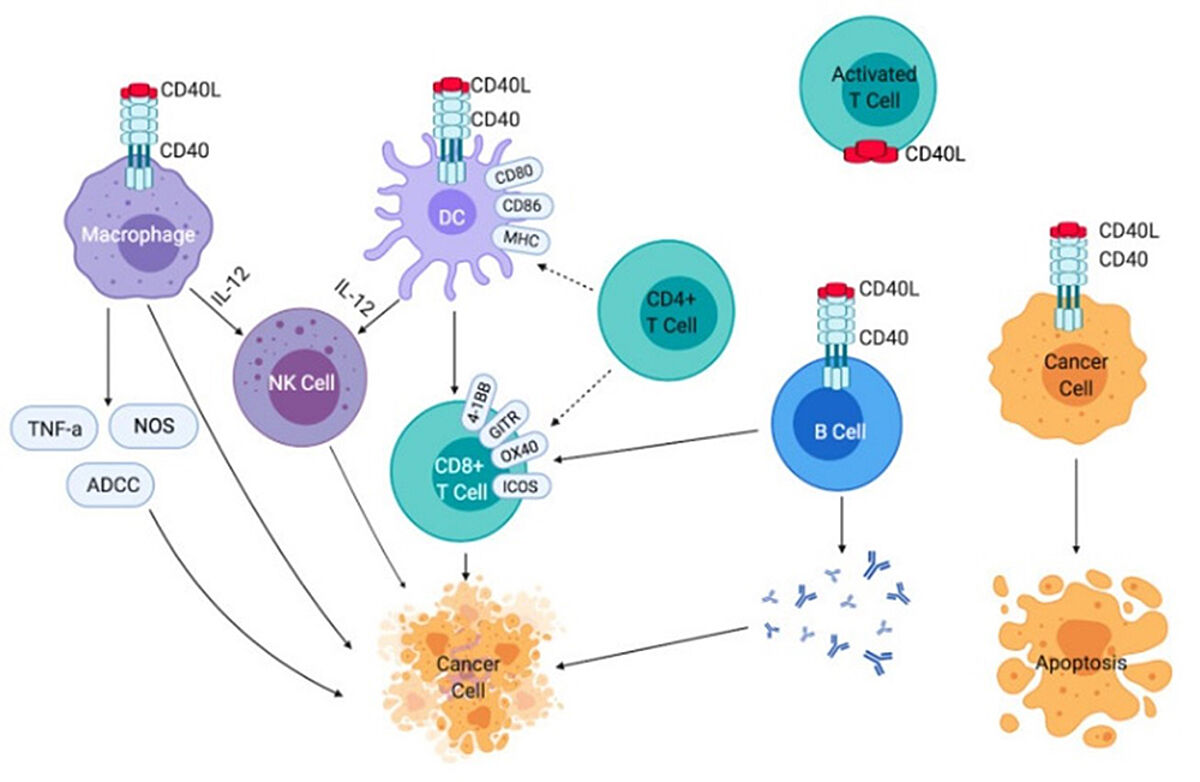
However, developing CD40-targeting IAAs has several challenges. The first of which is toxicity. CD40 is highly expressed across numerous cell types, thus leading to systemic inflammation throughout the body due to the increased immune response. Furthermore, certain CD40 IAAs have reported low levels of immune activation and antitumor efficacy, further emphasizing the need for personalized dosing to balance efficacy with toxicity.To address these challenges, researchers have explored other developmental strategies, such as Fc engineering, to enhance the binding of CD40-targeting IAAs. By improving the binding affinity to FcγRIIB, CD40 IAAs promote a higher order of crosslinking between the antibody and neighboring cells, therefore enhancing the clustering and signaling of CD40 around the target cell. Another more common strategy is the co-administration of therapies including other immune-based therapies (checkpoint inhibition, colony-stimulating factor 1 receptor (CSF-1R) inhibitors, and TLR3 agonists) and anti-angiogenic antibodies have been explored. This has shown to be effective in preliminary studies, combining CD40 agonist antibodies CP-870,893 with immune checkpoint inhibitor tremelimumab with a response rate of 27% in patients with advanced melanoma. 1
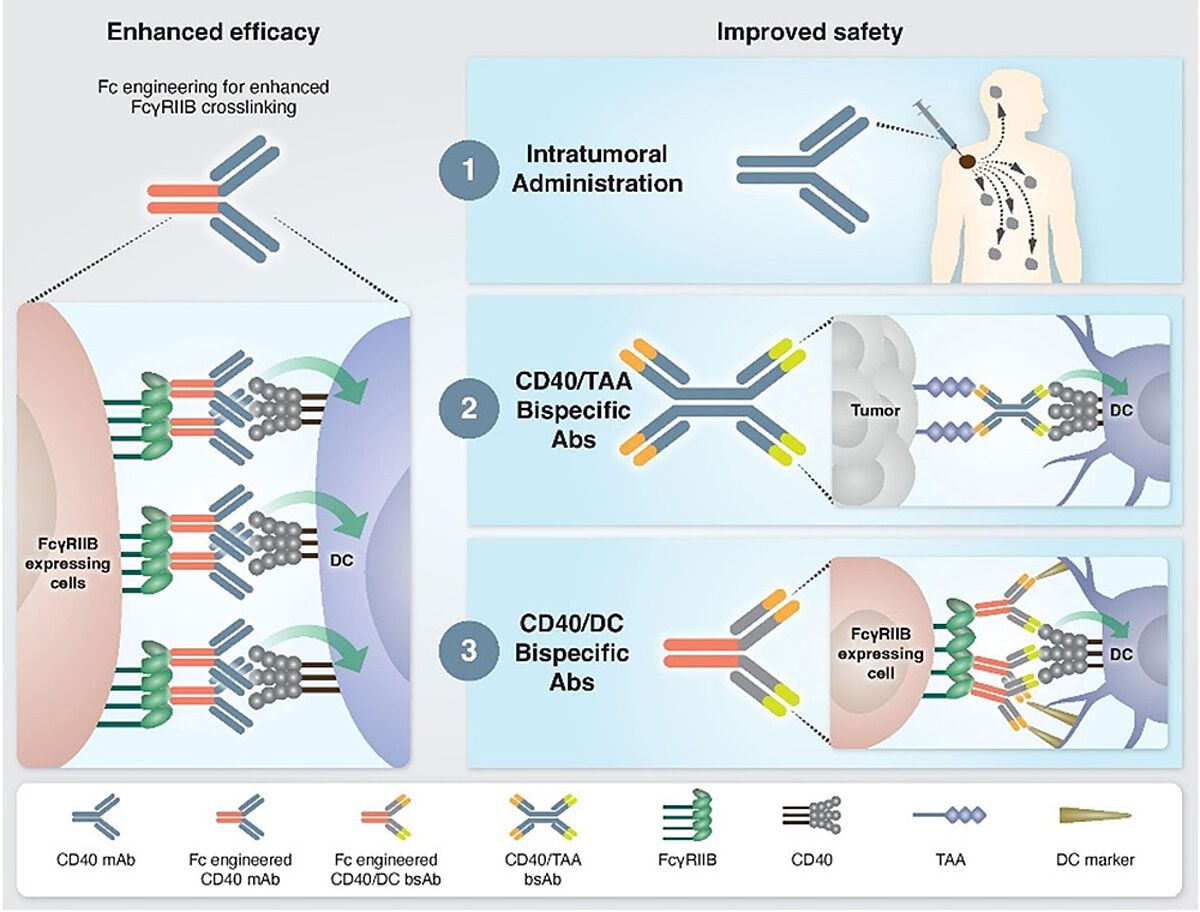
Overall, CD40 IAAs have immense potential in overcoming the challenges of checkpoint inhibitors. However, challenges such as toxicity and suboptimal efficacy need to be addressed, and combination therapy with other immunotherapies should be explored to enhance the efficacy of CD40 agonistic antibodies.
OX40 and its agonistic antibodies
OX40, also known as TNFRSF4 or CD134, is a co-stimulatory receptor found on the surface of a T cell that belongs to the TNF superfamily.3 OX40L, as the only ligand of OX40, is mainly expressed on activated antigen-presenting cells (APCs).3 When OX40 is activated by its natural ligand, OX40L, it enhances T cell activation, proliferation, and survival, leading to a stronger immune response against cancer cells. Moreover, high OX40 expression in the tumor immune infiltration has been used prognostically as a favorable prognosis for several types of cancer.3 Therefore, OX40 is widely regarded as one of the most promising targets for novel cancer immunotherapy.
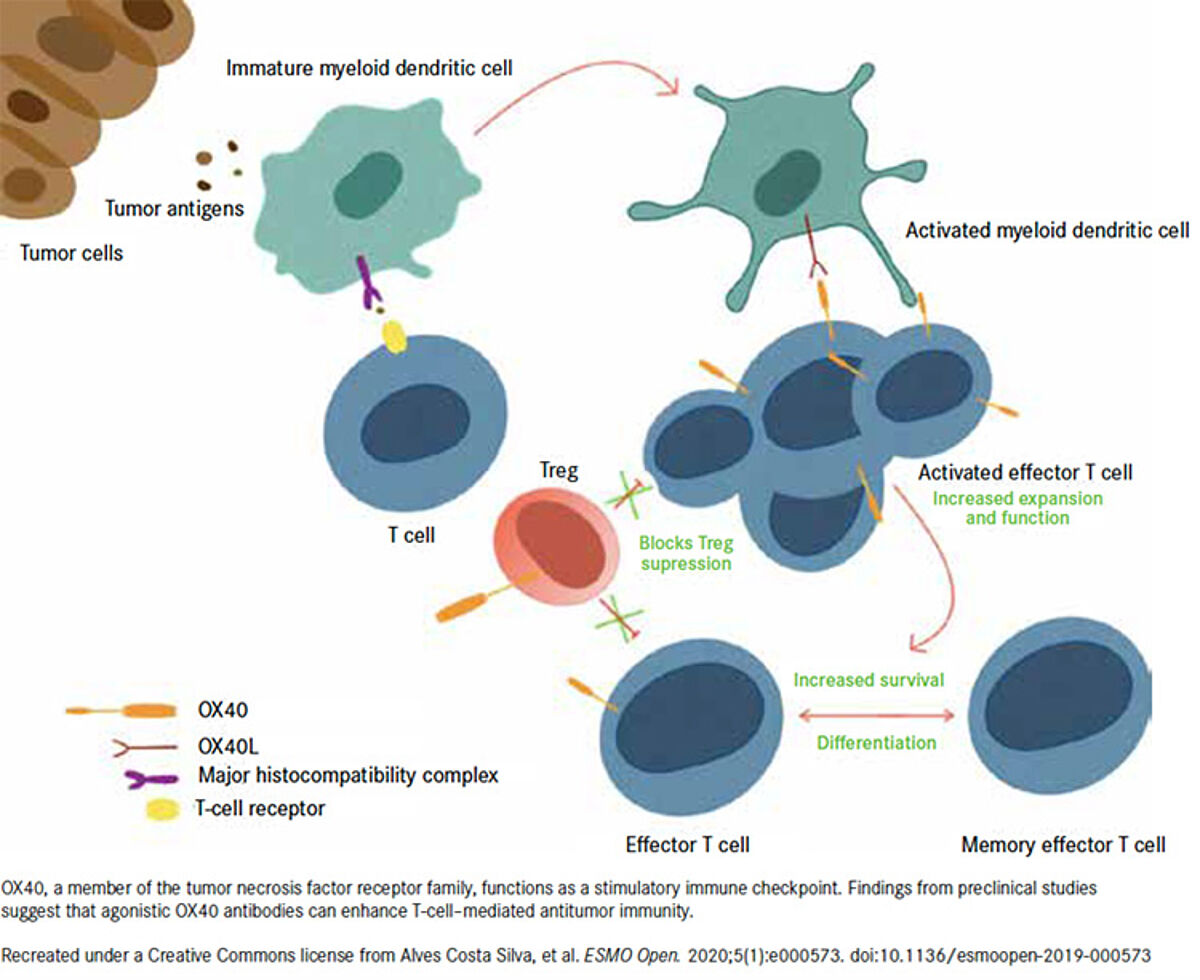
Various agonistic antibodies targeting OX40 have been developed and evaluated in early clinical trials, such as 9B12/ MEDI6469, MEDI-0562 (developed by AstraZeneca), 11D4 (PF-04518600, developed by Pfizer), and MOXR0916(developed by Roche).4,5 However, OX40 agonists as monotherapy have failed to live up to expectations, and several have been withdrawn due to modest antitumor activity.
Despite setbacks, there is still considerable interest in pursuing OX40-targeted drugs, but has focused on combination therapy, particularly with immune checkpoint inhibitors based on a preclinical demonstration of synergy.5 Roche's humanized effector-competent OX40 agonist antibody RG7888 (MOXR0916/pogalizumab) has completed preliminary toxicity testing in combination with the PD-L1 inhibitor atezolizumab in patients with advanced solid malignancies, and the combination was well tolerated with no dose-limiting toxicities. 4 Several clinical trials are exploring the application of costimulatory anti-OX40 mAbs as adjuvants in other therapies, such as anti-OX40 combined with chemotherapy and radiotherapy.
Designing the optimal OX40 agonist antibodies requires various important considerations such as binding affinity, epitope selection, valency, and receptor occupancy. One challenge inherent in developing agonist antibodies is that searching for the highest tolerable dose of receptor agonists has the potential to exhaust T cells and negate any antitumor efficacy.5 Additionally, since costimulatory receptor clustering is critical in signal activation, commonly induced by native ligand binding, designing an effective OX40 agonist antibody must be able to reproduce this effect.5 However, achieving this effect through enhancement of FcγR binding has resulted in the depletion of T cells due to another costimulatory agonist antibody. An alternative strategy explored previously is to increase valency. Inhibrx is developing INBRX-106, a hexavalent OX40 monoclonal antibody that can bind 6 OX40 receptors per molecule of drug. Bispecific antibodies, designed to engage two different targets, are also under study, such as ATOR-1015 targeting OX40 and the coinhibitory receptor CTLA-4 or F-star Therapeutics' FS120, a bispecific antibody that targets a second costimulatory receptor, 4-1BB (CD137), in addition to OX40. Shattuck Labs is pursuing a different drug design to achieve a similar effect to these bispecific antibodies, with their Agonist Redirected Checkpoint platform to create SL-279252, a bifunctional fusion protein that consists of the extracellular domains of OX40L and PD-1 joined by a central Fc domain.5
4-1BB and its agonistic antibodies
4-1BB, also referred to as CD137, is a co-stimulatory receptor that is expressed on the surface of activated T and B cells, monocytes, macrophages, dendritic cells (DCs), regulatory T cells (Tregs), and natural killer (NK) cells. CD137 can interact with TNFR-associated factor (TRAF) proteins, including TRAF1, TRAF2, and TRAF3, which activate different signaling pathways such as nuclear factor κB (NF-κB), MAPK, ERK, and JNK. 4
The activation of the NF-κB pathway promotes the upregulation of survival genes, such as Bcl-XL and Bfl-1, the downregulation of proapoptotic molecules like BIM, and transmission of signals that stimulate cell division. Additionally, the 4-1BB/4-1BB ligand (4-1BBL) signaling triggers biochemical signals that increase TH1 cytokines, including interleukin 6 (IL-6), IL-8, TNF, IL-12, and interferon γ (IFN-γ), suppress TH2 cytokines, potentiate activation, survival, proliferation, and cytotoxicity of T cells, increase IL-2 production, and promote the maturation of DCs.4
Several 4-1BB agonistic antibodies are currently under investigation in clinical trials for the treatment of various types of cancer, including urelumab (BMS-663513), PF-05082566, and utomilumab. However, their efficacy has been limited, and the clinical development of utomilumab was ultimately discontinued.6
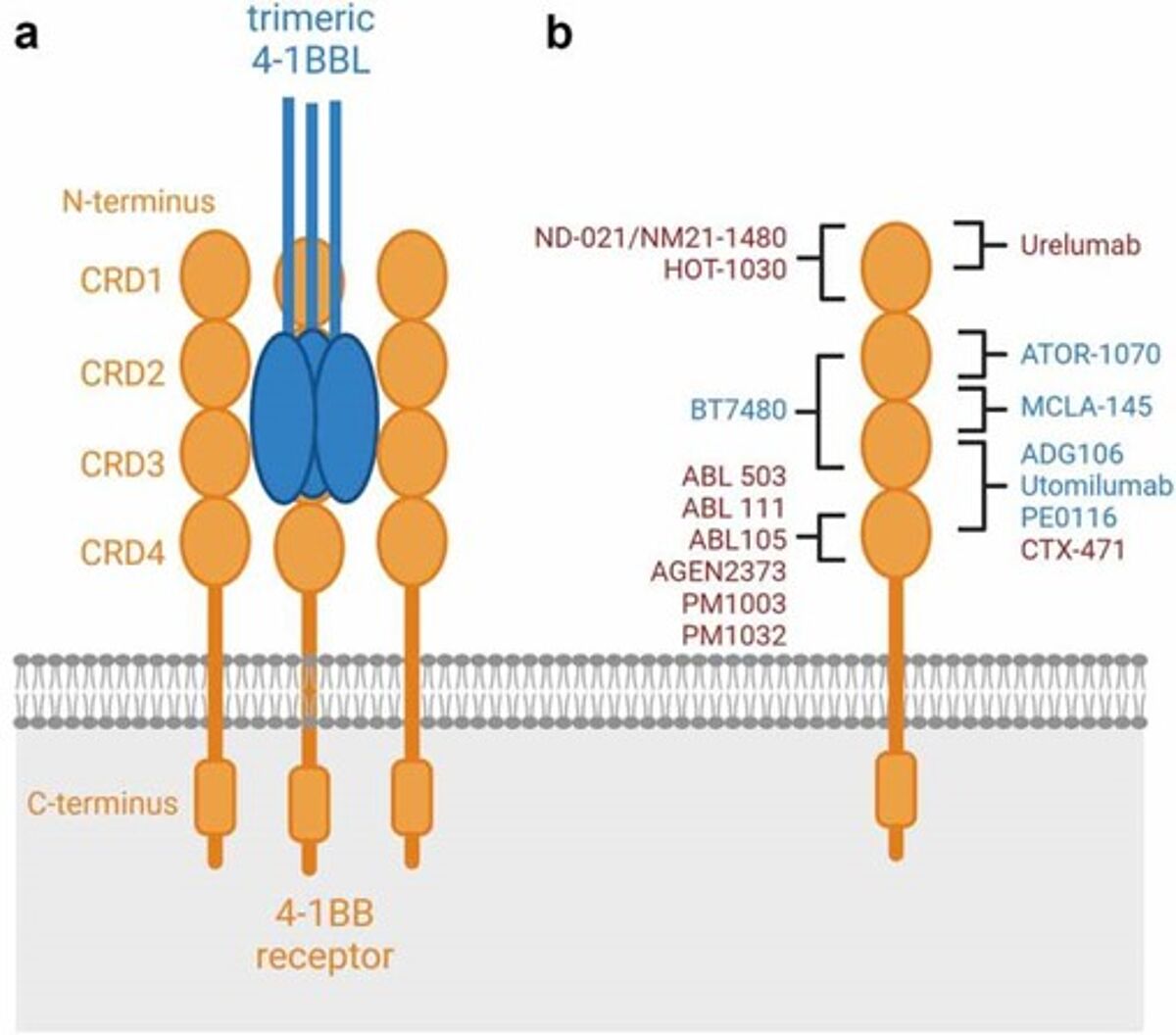
The first group of second generation of 4-1BB agonists utilize a human IgG1 or IgG4 scaffold and follows the mechanism of action of first-generation agonistic antibodies. These agnoists are highly dependent on FcγR crosslinking, however, they bind to different epitopes. However, a second group of 4-1BB agonists were designed to be polyvalent with bi-, tri- or tetra=- specific 4-1BB, thus a more comprehensive binding of 4-1BB while mediating 4-1BB hyperclustering. As such, These bi-, tri-, or tetra-specific agonists can display agonistic or inhibitory activity, leading to further anti-tumoral properties.6
Recently, researchers from Roche published a study in the journal Science Translational Medicine titled "A first-in-human study of the fibroblast activation protein-targeted, 4-1BB agonist RO7122290 in patients with advanced solid tumors." The study evaluated the safety and efficacy of RO7122290 in patients with advanced or metastatic solid tumors. The study found that the drug was generally well-tolerated, with only three dose-limiting toxicities reported. Moreover, eleven patients experienced a complete or partial response, suggesting the potential of RO7122290 in combination with atezolizumab or other immune-oncology agents for the treatment of solid tumors. However, further evaluation of RO7122290 is needed to confirm its efficacy and safety profile.7
Summary
Immune costimulatory agonists, including T-cell agonists, have shown great promise in cancer immunotherapy. While some agents have failed to fulfill their intended goals, overall, most antibodies have demonstrated promising results in early-phase trials. Combination therapy trials involving checkpoint inhibitors and chemotherapy or radiotherapy have also shown potential synergistic effects in inhibiting tumor progression. However, finding ways to manage immune-related adverse events, determining optimal treatment delivery methods, and discovering potential biomarkers to predict benefits from immune-agonist treatment are important challenges that need to be addressed. Despite these challenges, T-cell agonists are likely to be an important component of future cancer immunotherapy and are worth the effort to further monitor its research progress.7
Refrences
- Salomon Ran., Dahan Rony.(2022). Next Generation CD40 Agonistic Antibodies for Cancer Immunotherapy. Front Immunol, 13(undefined), 940674. doi:10.3389/fimmu.2022.940674
- Djureinovic Dijana., Wang Meina., Kluger Harriet M.(2021). Agonistic CD40 Antibodies in Cancer Treatment. Cancers (Basel), 13(6), undefined. doi:10.3390/cancers13061302
- Zhang Jing., Jiang Xiaoyong., Gao Han., Zhang Fei., Zhang Xin., Zhou Aiwu., Xu Ting., Cai Haiyan.(2022). Structural Basis of a Novel Agonistic Anti-OX40 Antibody. Biomolecules, 12(9), undefined. doi:10.3390/biom12091209
- Mascarelli Daniele E., Rosa Rhubia S M., Toscaro Jessica M., Semionatto Isadora F., Ruas Luciana P., Fogagnolo Carolinne T., Lima Gabriel C., Bajgelman Marcio C.(2021). Boosting Antitumor Response by Costimulatory Strategies Driven to 4-1BB and OX40 T-cell Receptors. Front Cell Dev Biol, 9(undefined), 692982. doi:10.3389/fcell.2021.692982
- Fresh Approaches May Be Key to Unlocking OX40 Checkpoint
- Claus Christina., Ferrara-Koller Claudia., Klein Christian.(2023). The emerging landscape of novel 4-1BB (CD137) agonistic drugs for cancer immunotherapy. MAbs, 15(1), 2167189. doi:10.1080/19420862.2023.2167189
- Melero Ignacio., Tanos Tamara., Bustamante Mariana., Sanmamed Miguel F., Calvo Emiliano., Moreno Irene., Moreno Victor., Hernandez Tatiana., Martinez Garcia Maria., Rodriguez-Vida Alejo., Tabernero Josep., Azaro Analia., Ponz-Sarvisé Mariano., Spanggaard Iben., Rohrberg Kristoffer., Guarin Ernesto., Nüesch Eveline., Davydov Iakov I., Ooi Chiahuey., Duarte José., Chesne Evelyne., McIntyre Christine., Ceppi Maurizio., Cañamero Marta., Krieter Oliver.(2023). A first-in-human study of the fibroblast activation protein-targeted, 4-1BB agonist RO7122290 in patients with advanced solid tumors. Sci Transl Med, 15(695), eabp9229. doi:10.1126/scitranslmed.abp9229
- Choi Yeonjoo., Shi Yaoyao., Haymaker Cara L., Naing Aung., Ciliberto Gennaro., Hajjar Joud.(2020). T-cell agonists in cancer immunotherapy. J Immunother Cancer, 8(2), undefined. doi:10.1136/jitc-2020-00096
Supplier

ACROBiosystems
ACROBiosystems is a leading manufacturer of recombinant proteins and other reagents to support the development of target therapeutics, vaccines, and diagnostics.
