Discover the tools behind breakthroughs
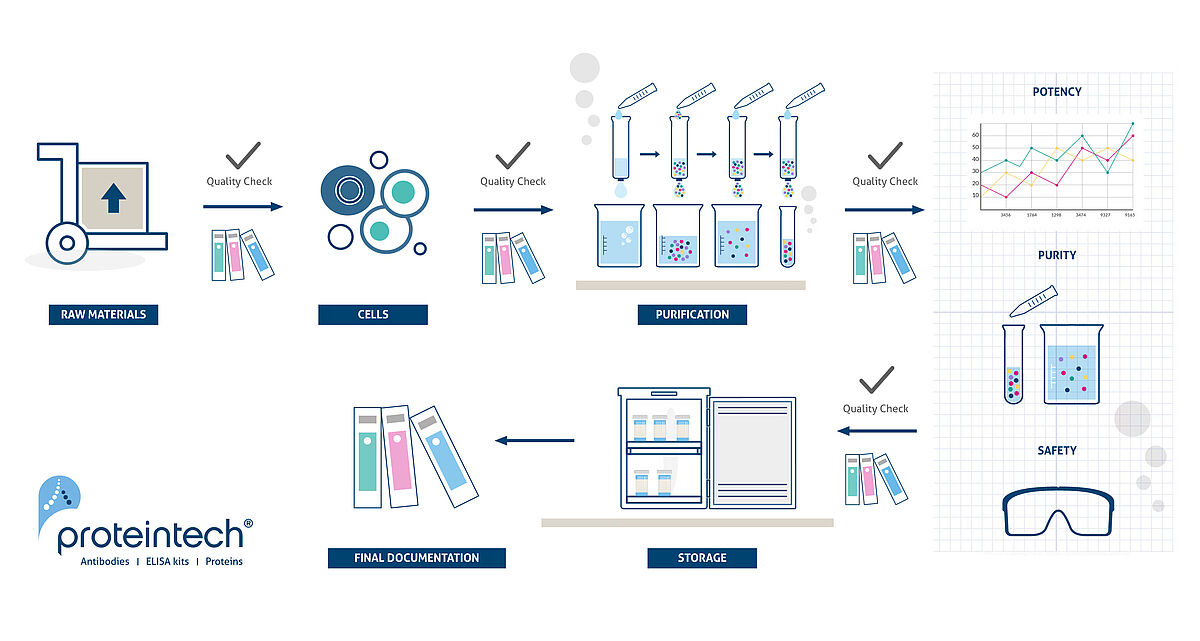
The entire line of HumanKine products is exclusively manufactured using a human expression system (HEK293), in accordance with USP and WHO standards, ensuring high bioactivity, stability, lot-to-lot consistency, and native human conformation & post-translational modifications. All Humankine® recombinant proteins are produced in Proteintech's in-house cGMP grade laboratory adhering to strict quality control regulations, ensuring maximum safety and reproducibility.
Why choose HumanKine cytokines & growth factors
- Protein is expressed in stable HEK293 system with optimized yields.
- Safety, efficacy, and lot-to-lot consistency for therapeutic manufacturing.
- Robust scalability from preclinical to clinical and commercial quantities.
- Seamless transition from research to GMP grade.
Is your focus more on GMP-grade products?
To facilitate a seamless transition from pre-clinical to clinical studies, both Research Grade and GMP Grade HumanKine cytokines and growth factors are manufactured through the same production process, minimizing variability in performance between Research Grade and GMP Grade products.
RUO to GMP, the same protein: the master cell line and manufacturing process are the same for GMP-grade and research-use-only cytokines and growth factors, which minimizes risk and compatibility testing and facilitates a seamless translation.
Well-defined media components and manufacturing processes ensure HumanKine products have minimal variability in their bioactivity and purity (close to 95%). Better lot-to-lot consistency means higher reproducibility in your results!
Request your free HumanKine sample
Get in touch today to find out what we can do for you. Request your free sample via the following form: