Phosphorylation, Ubiquitination, and Acetylation
The human genome is estimated to contain around 20,000 different genes, giving rise to approximately 80,000 transcripts and as many as 1 million different proteins1. The disparity between genome and proteome results from various genetic mechanisms and post-translational modifications (PTMs), which serve to increase functional diversity.
Mechanisms for protein diversity
Protein diversity is achieved through genetic mechanisms such as alternative transcription initiation, alternative splicing, and differential transcription termination, which generate multiple mRNA transcripts from a single gene. Further complexity is then introduced via post-translational modifications, which may occur at any stage in a protein’s life cycle and can impact a broad range of protein behaviors. Roles for PTMs include facilitating correct protein folding, directing protein localization, and controlling enzymatic activity, as well as promoting protein–protein interactions, enabling molecular trafficking, and instigating protein degradation. While the majority of PTMs are reversible, involving the covalent addition or removal of small chemical groups, others are permanent, including those based on proteolytic modification.
Types of PTMs
According to the dbPTM database, there are 76 different types of PTMs2. Of these, the 10 most widely studied PTMs are phosphorylation, ubiquitination, acetylation, N-linked glycosylation, methylation, 2-hydroxyisobutyrylation, O-linked glycosylation, succinylation, malonylation, and sumoylation, based on curation from over 40 external databases and more than 82,000 research articles3. A deeper delve into these data shows that the amino acids most commonly targeted for post-translational modification are serine, lysine, and threonine, followed by tyrosine, cysteine, and arginine. Disruption to normal PTM processes is implicated in conditions including cancers, metabolic syndromes, inflammatory disorders, type 2 diabetes, and neurodegenerative diseases4.
Major PTM types
While the high number of different PTM types prohibits a detailed review of every possible modification, we have covered the three most common PTMs here. A separate blog will discuss glycosylation and methylation.
Phosphorylation

Phosphorylation is the most well-studied PTM, with roles in many essential cellular processes. These include normal cell growth and division, apoptosis, and countless signal transduction pathways, of which the mitogen-activated protein kinase (MAPK) cascade, the mammalian target of rapamycin (mTOR) pathway, and the Janus kinase (JAK) signal transducer and activator of transcription (JAK-STAT) pathway are some of the best-known examples.
Phosphorylation is catalyzed by kinase enzymes, which transfer a phosphate group from ATP to amino acids with a hydroxyl group in their side chains (predominantly tyrosine, threonine, and serine residues). Dephosphorylation is catalyzed by phosphatases. The dbPTM databank shows approximately 1,900 phosphorylation sites to be associated with disease traits3. This equates to almost 80 kinase inhibitors having so far been approved for clinical use, mainly for the treatment of cancers5.
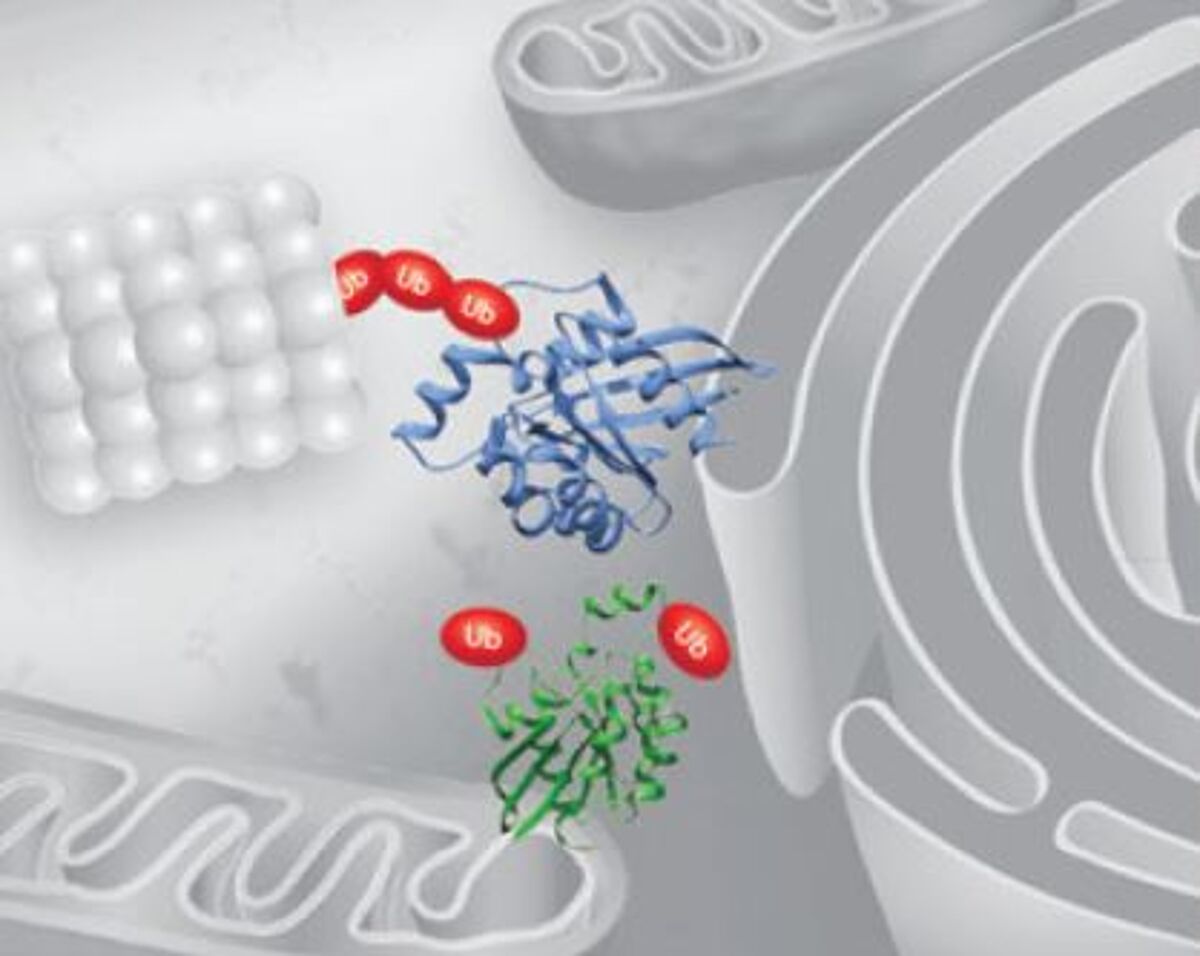
Ubiquitination
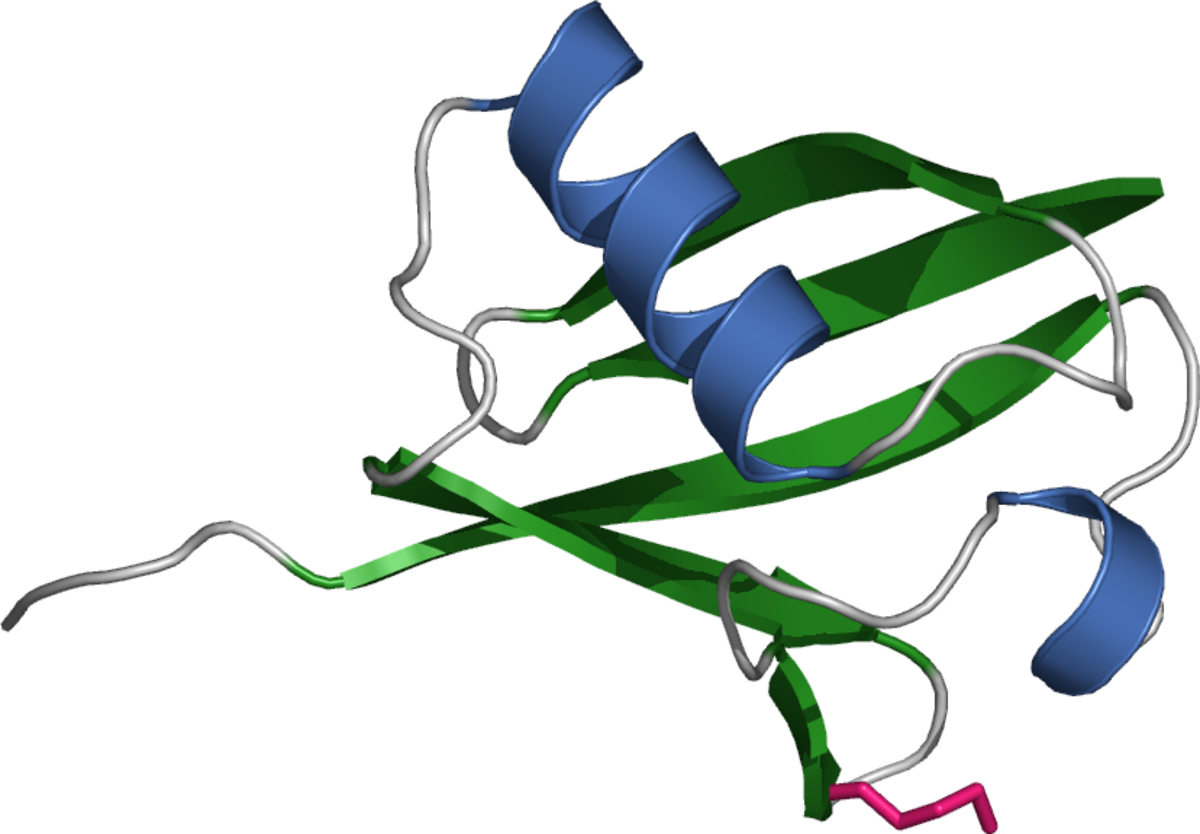
Ubiquitin is a 76 amino acid protein that can be attached singly to target molecules (monoubiquitination) or can form polymeric chains through ubiquitination of its seven internal lysine residues. While monoubiquitination is generally regarded as a signal for non-proteolytic events such as endocytosis, histone regulation, and DNA repair, polyubiquitination (especially Lys48- and Lys29-linked polyubiquitination) is associated with degradation of target proteins by the 26S proteasome6.
The ubiquitination process involves a 3-step enzymatic cascade, during which an enzyme complex containing ubiquitin-activating (E1), ubiquitin-conjugating (E2), and ubiquitin ligase (E3) enzymes attaches ubiquitin via its C-terminal glycine to the ε-amino group of a lysine residue on the substrate. The reverse reaction, ubiquitin removal, is performed by deubiquitinases. Dysfunction in the ubiquitin pathway has been linked to conditions including congenital developmental defects, cancer, and immune disorders.
Acetylation
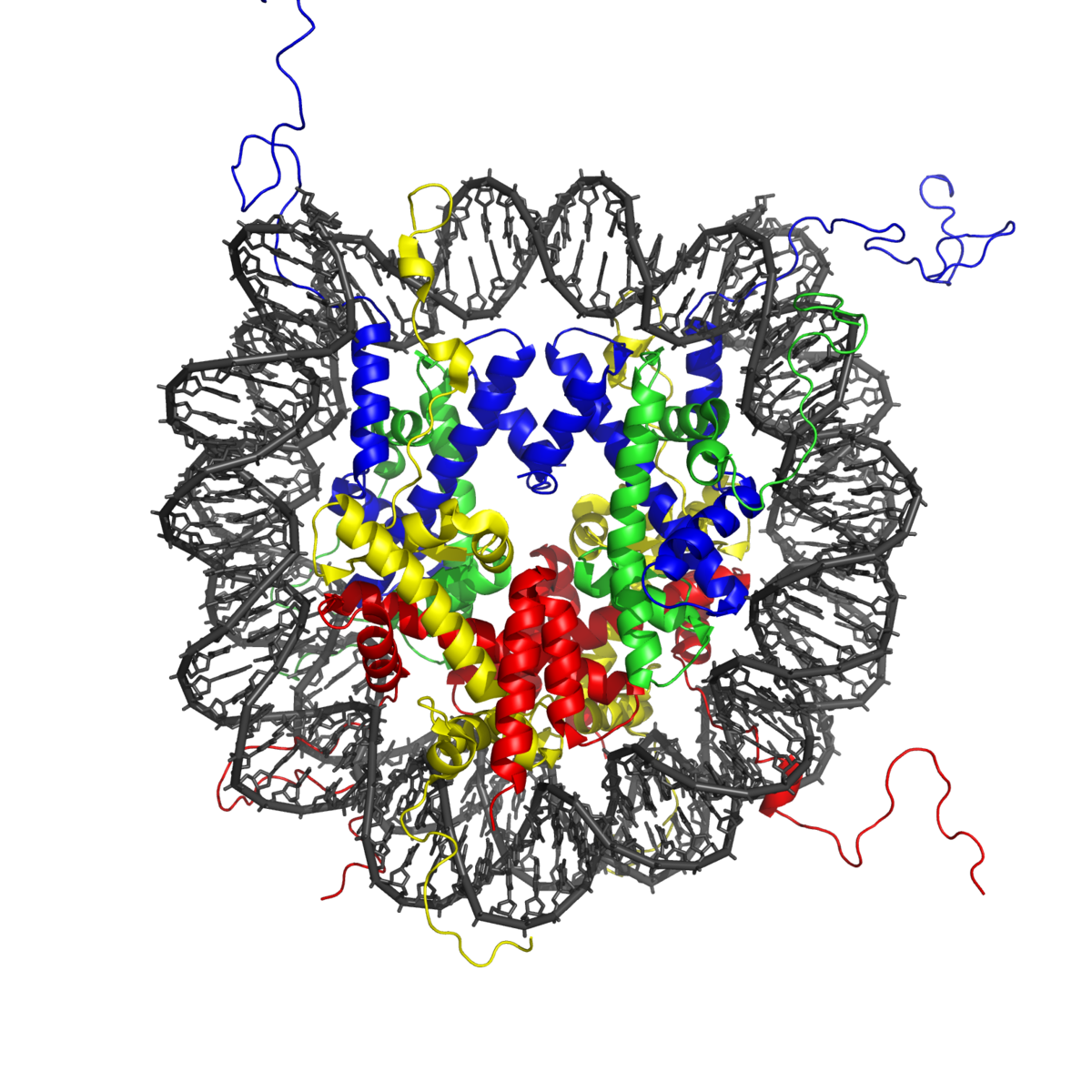
Acetylation is important for chromatin stability, protein–protein interaction, and cell cycle control, as well as for cell metabolism, nuclear transport, and actin nucleation4. However, a main reason for studying acetylation is to uncover its role in the histone code, a hypothesis which states that DNA transcription is largely regulated by post-translational modifications to histone proteins7.
Acetylation is catalyzed by lysine acetyltransferases (KATs), which use acetyl CoA as a co-factor for adding an acetyl group to the ε-amino group of lysine side chains. It is reversed by lysine deacetylases (KDACs). Dysregulated acetylation has been associated with cardiovascular disease, neurodegenerative disorders, and cancer. In addition, it was recently discovered that SARS-CoV-2 nucleocapsid proteins are strongly acetylated by human acetyltransferase enzymes, which may influence their functions8.
LubioScience represents some of the most trusted brands in research and works closely with our partners to offer high-quality reagents for studying PTMs including phosphorylation, ubiquitination, and acetylation. Contact us today to discuss how we can support your project.
Refrences
- https://www.nextprot.org
- https://awi.cuhk.edu.cn/dbPTM/
- https://pubmed.ncbi.nlm.nih.gov/34788852/
- https://pubmed.ncbi.nlm.nih.gov/33826699/
- https://pubmed.ncbi.nlm.nih.gov/34002056/
- https://pubmed.ncbi.nlm.nih.gov/22357635/
- https://pubmed.ncbi.nlm.nih.gov/10638745/
- https://pubmed.ncbi.nlm.nih.gov/33894414/
