Cell death is a seemingly simple concept—the irreversible loss of cellular function and/or physical integrity. Yet, death may claim cells in manifold ways and for various reasons. The idea of the death of a cell as an inevitable component of a living cell is likely as old as the concept of cellular life itself. However, the concept of controlled and orchestrated cell death managed by dedicated cellular mechanisms was established only a few decades ago. The first morphological descriptions of it date back over one-and-a-half century ago. Since then, our understanding of the complexity of the ways and forms in which cellular demise may manifest itself has continuously gained momentum over the recent decades in classical "the more we learn, the more we know what we don't know yet" fashion.
Initially, cell death was mainly described and classified based on its various morphological appearances. In 1972, Kerr, Wyllie, and Currie coined the term 'apoptosis' (a term derived from the ancient Greek meaning 'falling off') to describe a morphologically stereotyped form of cellular demise with characteristic morphological features such as chromatin condensation, cell shrinkage, nuclear fragmentation, and final membrane blebbing, ultimately resulting in cell fragments with intact membranes, so called apoptotic bodies (1). Soon after, a classification of cell death emerged that discriminated between type I or apoptosis as described above, type II or autophagy, characterized by extensive vacuolization of the cytoplasm, and type III or necrosis, resulting in cellular lysis with neither of the above described features. This classification has dominated our understanding of cell death for decades, despite increasing awareness of indisputable discrepancies between the emerging knowledge about the involved molecular mechanisms and the resulting morphological appearance.
Furthermore, it became increasingly evident that many forms of cell death can present both apoptotic and necrotic traits. Thus the established classification on morphology alone does not clearly distinguish cell death forms. It does not even reliably discriminate between accidental cell death (ACD), the instantaneous and catastrophic demise of cells as a consequence of physical, chemical, or mechanical insults, and regulated cell death (RCD), which relies on dedicated cellular and molecular machinery. Nonetheless, despite its shortcomings, the morphological classification described above is still widely in use.
RCD serves to eliminate superfluous, irreversibly damaged, and/or potentially harmful cells. Elimination of cells is beneficial in two diametrically opposed scenarios. On the one hand, eliminating excess cells serves many physiological functions in development and homeostasis. Physiologic RCD in the absence of any exogenous perturbations is often denominated as programmed cell death. However, the term is also confusingly used to describe apoptosis alone or regulatory cell death in general. On the other hand, stress-driven RCD serves to eliminate cells that have been severely compromised by extracellular perturbations or pose a danger to their environment or organism, for example, due to pathogens or DNA damage beyond repair.
Metabolic activity. Probably the most intuitive parameter one can think of to understand if the cells are alive and in good conditions. It can be approached from different angles.
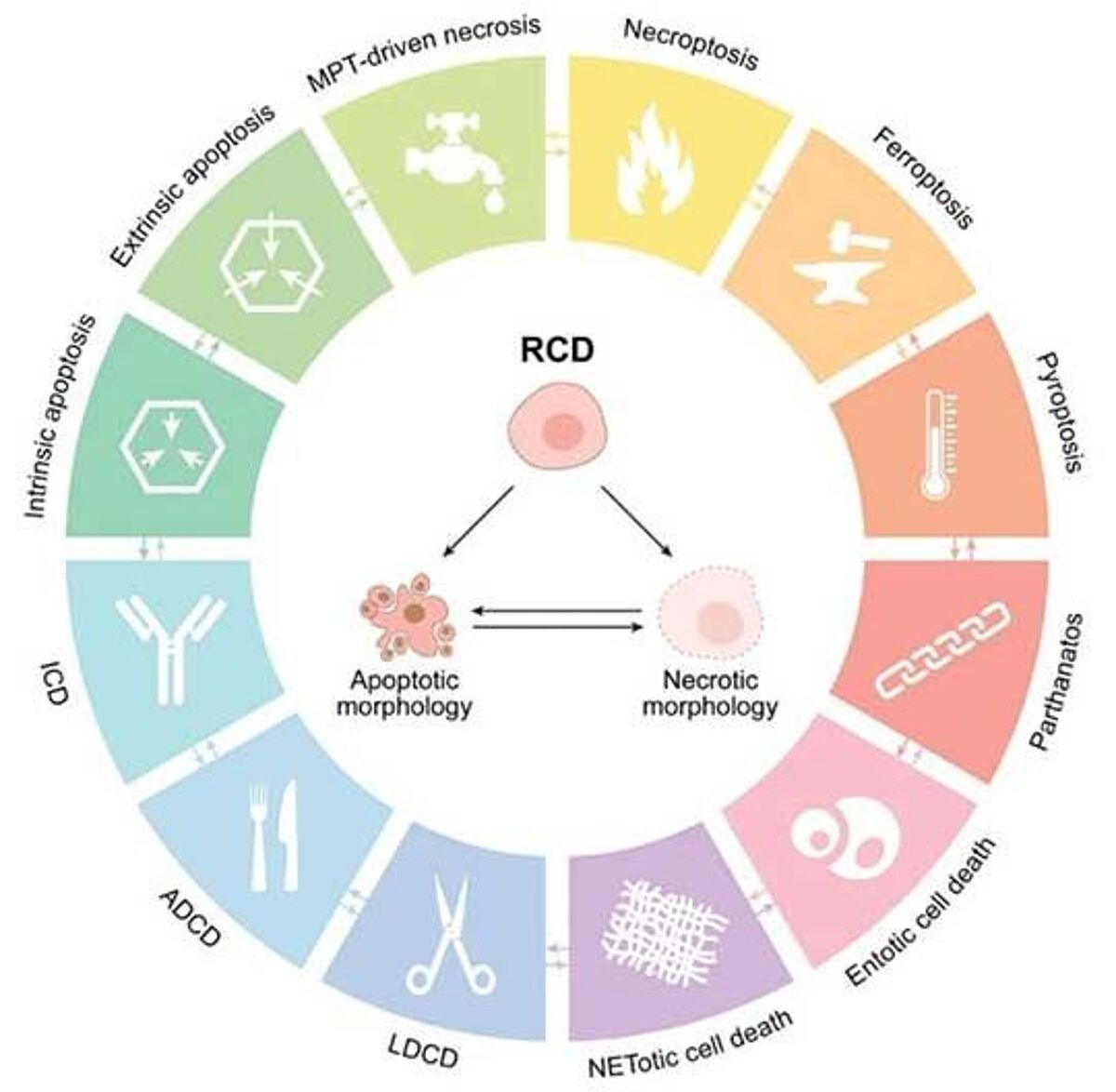
Various mechanisms to orchestrate cellular demise have developed to fulfill the various demands and functions that RCD has evolved to serve. The Nomenclature Committee on Cell Death (NCDD) recognizes over a dozen distinct forms of RCD (2), of which the main ones are briefly introduced here:
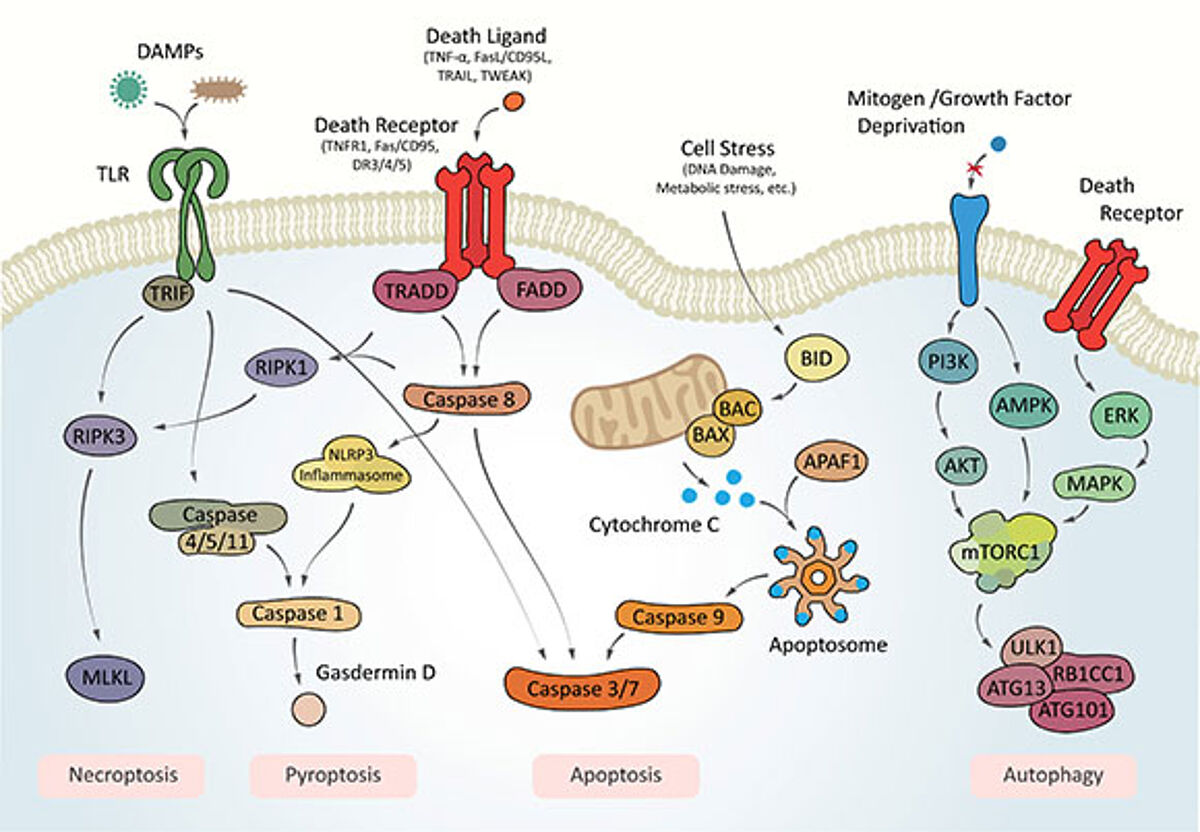
Intrinsic apoptosis is a form of RCD triggered by various microenvironmental perturbations, such as growth factor withdrawal, mitotic defects, DNA damage, endoplasmic reticulum, mitochondrial, replicative, or oxidative stress. Intrinsic apoptosis is critically triggered by irreversible and widespread mitochondrial outer membrane permeabilization. Release of cytochrome C into the cytoplasm leads to the formation of the apoptosome, evoking downstream effector caspases in a classical caspase cascade, which leads to cell and nuclear shrinkage, followed by fragmentation and ultimately efferocytosis – the clearance by phagocytic cells.
Extrinsic apoptosis is morphologically indistinguishable from intrinsic apoptosis as it relies mainly on the same downstream effector mechanisms but is triggered by perturbations of the extracellular microenvironment. Extrinsic apoptosis is mostly elicited by death receptor activation through death ligands, but can also be activated by the signal withdrawal of dependence receptor signaling. Death receptors such as Fas/CD95, TNFR1, or DR3/4/5 are bound by their respective ligands, which typically leads to trimerization and the respective associated death domain (e.g., FADD, TRADD), and subsequent association and cleavage-mediated activation of caspase 8 (or 10), ultimately triggering the effector caspase cascade.
Autophagy-dependent cell death is a form of RCD in which the autophagic machinery breaks down most of a cell's contents and components. Autophagy-dependent cell death is distinctive from enhanced autophagy activity contributing to other forms of RCD, such as Fas-mediated extrinsic apoptosis, necroptosis, or ferroptosis.
Necroptosis is a form of RCD triggered by specific death receptors or pathogen recognition receptors, such as Toll-like receptors, which activate a downstream effector pathway, resulting in a very rapid form of RCD with a necrotic morphotype.
Mitochondrial permeability transition (MPT)-driven necrosis is a form of RCD caused by severe intracellular perturbations, such as excessive oxidative stress or Ca2+ overload, and normally manifests in a necrotic morphotype.
Pyroptosis is a form of RCD triggered by perturbations related to innate immune system activities and manifests itself with a distinct morphotype. Characteristic is a peculiar form of chromatin condensation accompanied by cellular swelling culminating in cell membrane rupture.
Ferroptosis is a form of RCD that occurs independent of caspases, necrosome, or autophagy pathways, and manifests with a necrotic phenotype. Ferroptosis is triggered by specific perturbations within the intracellular domain, notably severe lipid peroxidation, which relies on the generation of reactive oxygen species (ROS) and iron availability.
Entotic cell death is a form of cellular cannibalism, where non-phagocytic cells engulf neighboring cells and digest them. It often occurs in malignant tissues.
Cell Death Research at Enzo
Enzo Life Sciences offers a comprehensive product portfolio for Regulatory Cell Death Research. At Enzo, we provide scientists with a broad selection of unique tools to detect and discriminate RCD, or study caspases or death receptor signaling. For example, our SUPERFASLIGAND® is one of the most effective ways to activate Fas-signaling and Fas-dependent RCD and has been used in a recent study to understand the transition from Caspase-1 mediated necrotic pyroptosis to apoptosis in the absence of Gasdermin D (3). Furthermore, our GFP-CERTIFIED® Apoptosis/Necrosis detection kit, which allows efficient discrimination between apoptotic and necrotic morphotypes of cell death, has recently been used successfully to monitor effects or the inhibitory role of TRIP-Br1/XIAP in necroptosis (4).
Do you have any questions regarding our products or their implementation into your research? Don't hesitate to contact our Technical support team for more information. We will be happy to assist.
Author: Hartmut Pohl
Supplier

Enzo Life Sciences
Enzo Life Sciences is a leading life sciences and biotechnology company focused on harnessing genetic processes to develop research tools. The range includes assay kits, imaging probes, antibodies, proteins and small molecules.
About Enzo Life Sciences Shop for Enzo Life Sciences products
