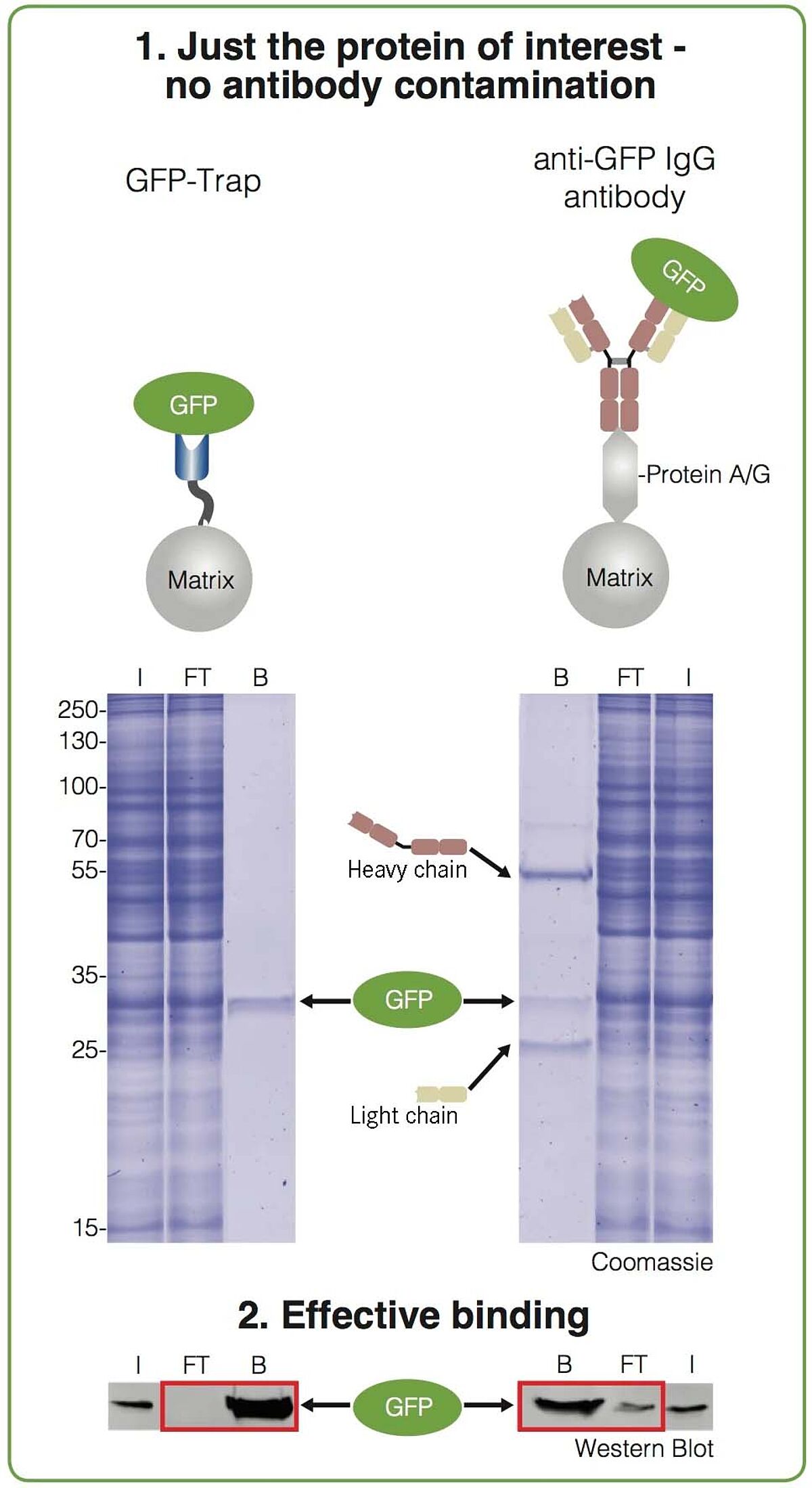
1. CLEAN—No contamination by heavy & light chains
As the anti-GFP nanobody consists of a single variable domain, you don’t have to worry about any contaminating light and heavy chains. This is illustrated in the figure where you can see the comparison between using GFP-Trap and an anti-GFP IgG antibody coupled to Protein A/G. With the IgG antibody, two additional strong bands besides the purified protein of interest are visible in the bound fraction. These bands can be a serious problem if you want to detect proteins of a similar size.
2. PURE—Very low background
GFP-Trap is stable in harsh and stringent conditions. This allows you to set the binding condition so that only the protein of interest is bound. ChromoTek lists the following conditions that can be applied to their GFP-Trap:
- up to 83°C
- 1mM DTT (10mM for GFP-Trap Magnetic Particles)
- 2mM TCEP
- 3M Guanidinium•HCl
- 8M Urea
- 2M NaCl
- 2% Nonidet P40 Substitute
- 1% SDS (0.2% for GFP-Trap Magnetic Particles)
- 1% Triton X-100
3. FAST—Complete the workflow in 1 hour
GFP-Trap is ready to use and the cell-lysate incubation time typically lasts between 10 and sixty minutes (instead of overnight). This extremely short incubation time is possible thanks to GFP-Trap’s high sensitivity, fast binding and low dissociation constant. Additionally, if you have to perform Co-IP, less stable protein interaction partners are more likely co-precipitated due to shortened processing time.
4. RELIABLE—Strong binding
The anti-GFP VHH in GFP-Trap has a very specific and strong binding to GFP-fusion proteins with a dissociation constant of KD = 10-12 M (kon = 2.6 *107 1/Ms, koff = 3.03*10-5 1/s).
5. VALIDATION—Characterization of function and structure
One of the qualities of ChomoTek is that they really invest in the characterization of their products. As mentioned above, the kinetics have been measured. In addition, the crystal structure of anti-GFP VHH is available and its function has been tested in many applications (IP, Co-IP, affinity purification, mass spectrometry, enzyme activity measurements, RIP analysis).
GFP-fusion proteins have been analyzed using GFP-Trap for many different cells and species. These include (but are not limited to!) Aspergillus fumigatus, Arabidopsis thaliana, Drosophila melanogaster, E. coli, Human cell lines, Mus musculus, Oryza sativa, Plasmodium berghei, Petunia inflata, Rice protoplast, Saccharomyces cerevisiae.
6. TRUST— Constant high quality
As the anti-GFP VHH is recombinantly expressed in bacteria, you can count on almost no lot-to-lot variation. In addition, ChromoTek has a stringent quality control and a dedicated QC/QM laboratory since 2015.
7. CONFIDENCE—Highly cited
It is not without reason that GFP-Trap is considered the gold-standard for the immunoprecipitation of GFP-fusion proteins: GFP-Trap is the most frequently cited monoclonal anti-GFP antibody! Also, ChromoTek’s GFP-Trap has over 1’000 peer-reviewed articles with references.
For a recent example in a high impact publication, check out Daly et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. Science. 2020 Nov 13;370(6518):861-865. doi: 10.1126/science.abd3072. Epub 2020 Oct 20. PMID: 33082294. Read a summary of this publication on ChromoTek’s blog.
Ask us for a sample