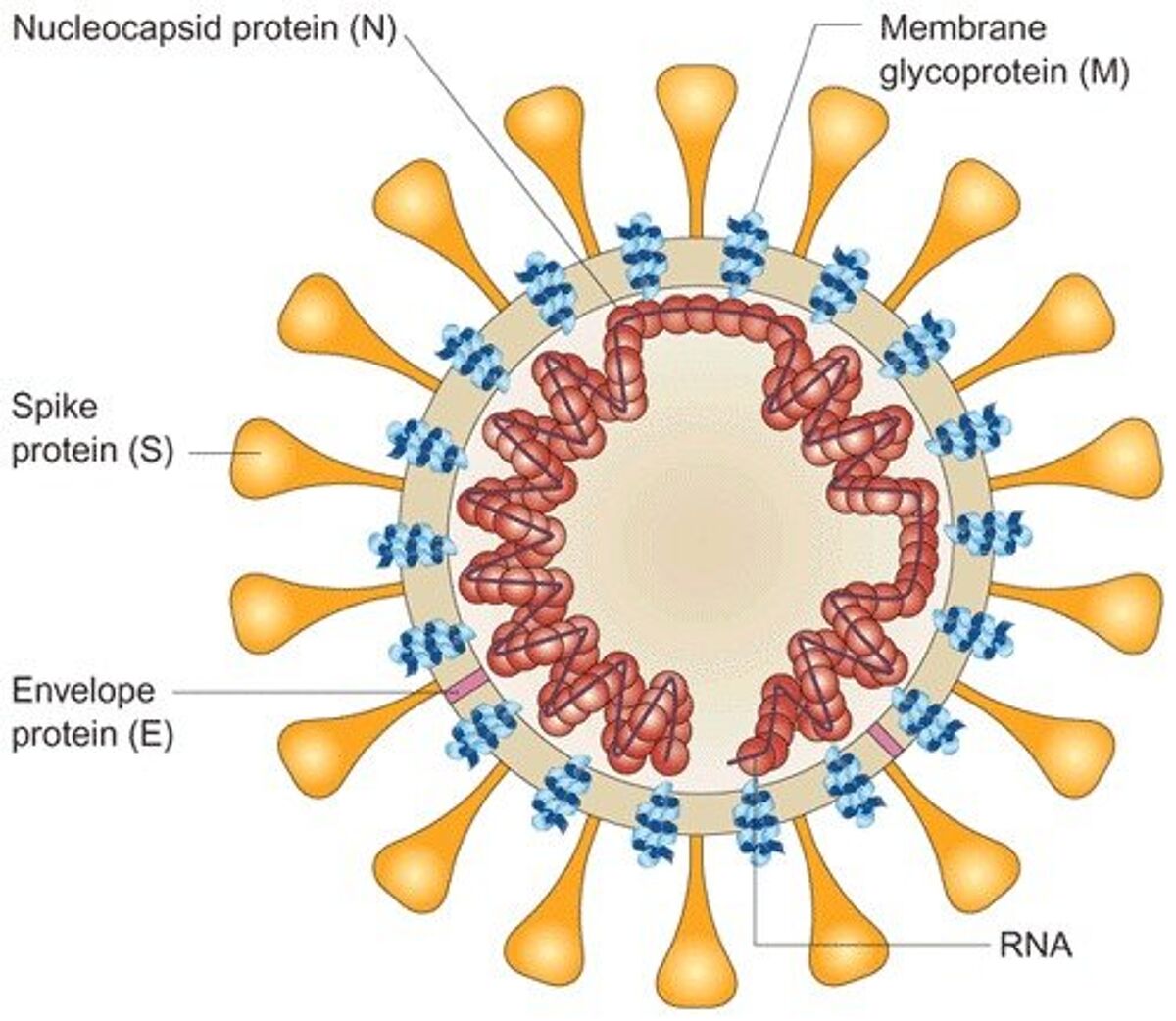
The glycosylated surface spike protein of SARS-CoV-2 mediates the viral entry into the host cells and currently is the main focus for approaches to neutralize coronavirus entry. For this reason, the trimeric SARS-CoV-2 spike (S) protein is a preferred target for screening of specific neutralizing antibodies in sera of seroconverters or investigation of synergistic effects of neutralizing antibodies.
In a recently article published by Huo et al. (1) the neutralizing effect of antibody clone CR3022 has been described as a consequence of the destruction of the prefusion spike trimer conformation. CR3022 binds a highly conserved, allosteric binding site of the SARS-CoV-2 receptor binding domain (RBD), which is different from that used by the ACE2 receptor. The induced conformational changes interfere with an S2-mediated membrane fusion and entry into the host cell.
Antibody clone CR3022 is a suitable positive control in serological assays to detect antibodies in human serum that bind SARS-CoV-2 S-protein (2,3). It has therefore been specualted that CR3022 has the potential to be developed into a prophylactic or therapeutic agent, alone or in combination with other neutralizing antibodies, for treatment of SARS-CoV infections (4).
Browse recombinant CR3022 antibodies
Learn more about our new recombinant trimeric spike protein
Literature
(1) Huo et al. Neutralization of SARS-CoV-2 by Destruction of the Prefusion Spike. Cell Host & Microbe 28, 1–10 (2020)
(2) Amanat, F., Stadlbauer, D., Strohmeier, S. et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nature Medicine (2020).
(3) Stadlbauer, D. et al. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 57, e100 (2020).
(4) Tian, X. et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes Infect. 9, 382–385 (2020).