Recombinant Antibodies: Benefits & Methods for Discovery
Antibody discovery is fundamental to a broad range of research and clinical applications. Critically, developing new recombinant antibodies promises improved experimental reproducibility as well as provides opportunities for engineering.
What are recombinant antibodies?
Commercially available antibodies for scientific research have traditionally been categorized as either polyclonal or monoclonal. The main functional difference between these is the number of epitopes each antibody preparation will bind. While polyclonal antibodies target multiple epitopes, which can provide signal amplification, monoclonal antibodies recognize just a single epitope, meaning they yield more reproducible results. More recently, these formats have been complemented by a rapidly growing number of recombinant antibodies – namely, antibodies that are defined by a specific protein sequence. Recombinant antibodies offer a wealth of advantages, including greater consistency; faster, more scalable production; and the capacity for engineering to support unmet experimental needs.

How are recombinant antibodies produced?
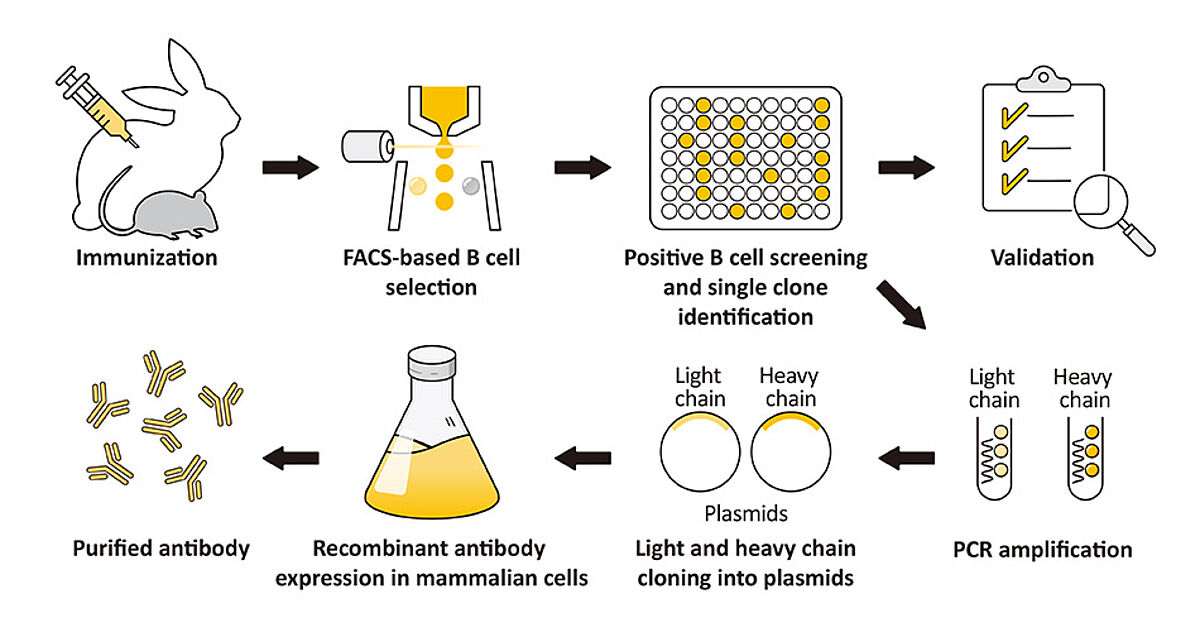
One method for producing recombinant antibodies involves sequencing existing clones and integrating the antibody-coding DNA into an expression vector. This is then introduced into a mammalian cell line (usually a suspension HEK or CHO line to maintain essential post-translational modifications) for expansion in culture. Alternatively, recombinant antibodies can be produced using display technologies, typically phage display, which begins with creating a library of antibody fragments (e.g., scFv, Fab, or VHH) from a suitable host species. To achieve this, the mRNA is isolated from either naïve or immune B lymphocytes and used for cDNA synthesis; the antibody-coding genes are then amplified and cloned into phagemid vectors for biopanning against the target antigen. Once single phage binders have been identified and characterized for specificity and affinity, the DNA is extracted for antibody sequencing and expression in mammalian cells.
Why use recombinant antibodies?
While traditional polyclonal and monoclonal antibodies remain highly popular, the use of recombinant antibodies is increasing. A main reason for this is the well-documented reproducibility crisis, which has led both researchers and publishers to demand greater clarity regarding antibody provenance. Additionally, doubt over the true monospecificity of conventional, hybridoma-derived monoclonals has fueled recombinant antibody use as a means of avoiding reduced specificity and assay signal. Other reasons for using recombinant antibodies include their superior batch-to-batch consistency, guaranteed long-term supply, and the fact that production methods are both faster and avoid the need for animal involvement. Until recently, a viable alternative to using polyclonal antibodies for signal amplification was not available; however, recombinant oligoclonal antibodies (mixtures of recombinant monoclonals, each recognizing a different epitope on the same target antigen) are now being developed. Recombinant antibodies are also amenable to engineering, allowing them to be adapted to meet different needs.
Increased antibody functionality through engineering
Recombinant antibodies can be engineered to support many different applications. For research use, a tried and trusted method involves introducing point mutations into the antibody Fc region to prevent interaction with Fc receptors expressed on the surface of immune cells. As well as reducing non-specific background signal in techniques such as flow cytometry and immunohistochemistry (IHC), this abrogates the antibody directed cytotoxicity (ADCC) effector function in vivo. Another strategy is to switch the Fc region of an antibody to a different species, isotype, or subclass; this increases flexibility for co-immunostaining applications and can also prevent unwanted immunogenicity during in vivo studies. For example, switching the Fc portion of a rat antibody that will be used for murine in vivo research to a mouse Fc reduces the risk of unwanted cross-reaction. Additionally, by producing antibody fragments rather than intact antibody molecules, it is possible to enhance tissue penetration for detection of less accessible epitopes. Therapeutic antibody engineering approaches include humanization (engineering antibodies from non-human hosts to make them more similar to human antibodies) to improve in vivo tolerance and minimize the production of anti-drug antibodies; and generation of bispecific antibodies, such as those that can simultaneously bind tumor cell receptors and cytotoxic immune cells.
Validation is imperative
Irrespective of whether a polyclonal, monoclonal, or recombinant antibody is chosen, validation is critically important. While an antibody manufacturer should offer proof of antibody specificity and its suitability for use in different applications, the onus is on the end user to further validate the antibody in their own model system. In-house validation should employ suitable controls to optimize the antibody concentration and should, ideally, combine multiple testing strategies to build confidence in antibody performance.
Read more about
Recombinant Antibody Applications
Discover our
Custom Antibody Development and Production Services
LubioScience represents some of the most trusted brands in research and our partners include several leading players in the field of recombinant antibody production. Contact us today to discuss how we can support your project.
Selected Suppliers for recombinant antibodies:

GeneTex’s rAb protocol employs a multi-parameter FACS-based approach to isolate antigen-specific IgG+ memory B cells from an immunized animal. Once cloned, the supply of a given rAb is inexhaustible with exceptional reproducibility.

Absolute Antibody offers a wide range of human-derived antibodies specific to viral pathogens or self-antigen. The proprietary cloning platform allows to produce high-quality recombinant versions of these antibodies.
See Absolute Antibody's recombinant antibodies

Bethyl has applied B-cell sorting and recombinant DNA technology to deliver high quality recombinant rabbit monoclonal antibodies (RmAbs). Their stringent validation ensures that the antibody is on target and will work in the applications stated.
