Endotoxin contamination of plasmid DNA
Endotoxin contamination is a common problem with nucleic acids and recombinant proteins that have been purified from gram-negative bacteria such as E. coli. Endotoxins, also known as lipopolysaccharides (LPS), are the major component of the outer membrane of these Gram-negative bacteria.
When Gram-negative bacterial cells are lysed due to death or during growth and division, high concentrations of endotoxins are subsequently released. A single E. coli contains about 2 million LPS molecules per cell. In addition, endotoxins are highly heat-stable and are not destroyed in sterilizing conditions. This makes managing or removing endotoxin contamination in cell cultures or purified DNA preparations extremely difficult.
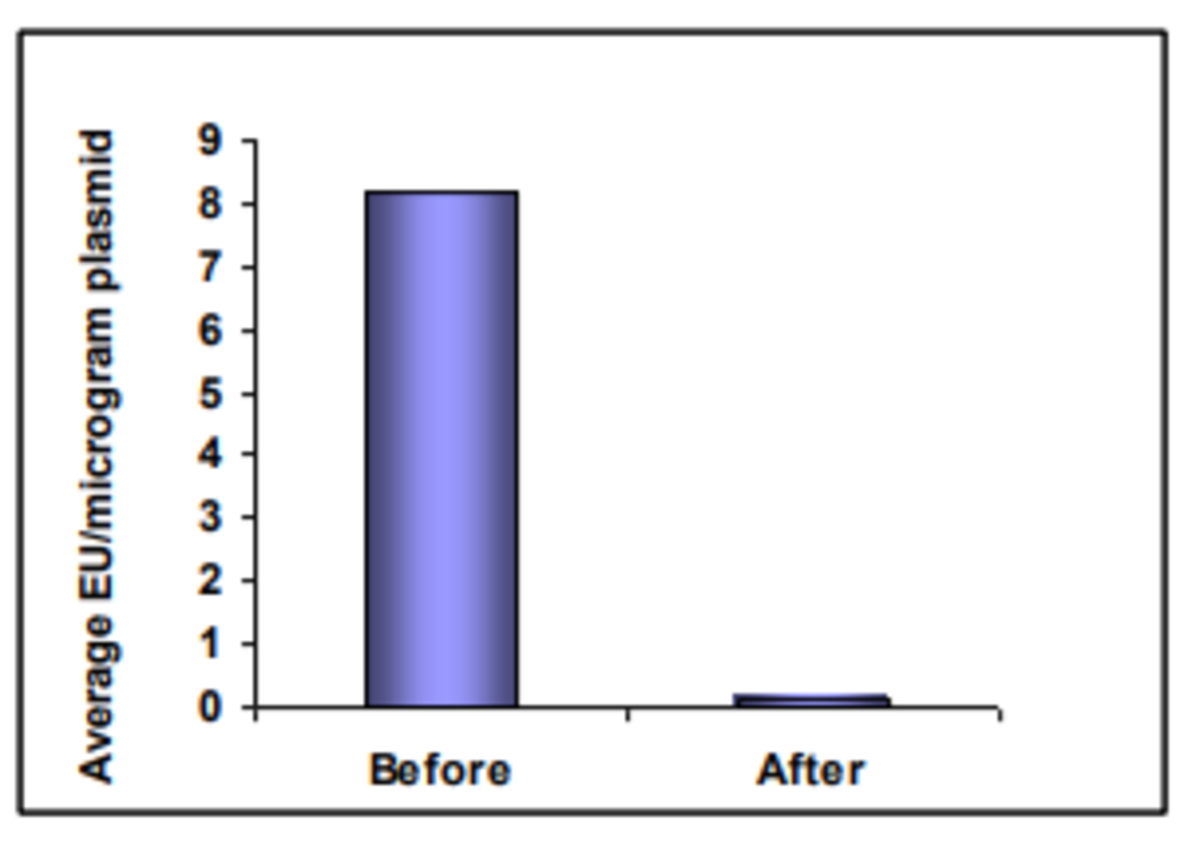
Endotoxins are a common contaminant in nucleic acid preparations, particularly when purified from plasmid DNA. Endotoxins co-purify with plasmid DNA due to their size and consequently, high levels of endotoxins can contaminate purified plasmid DNA, affecting subsequent applications using the plasmid DNA. High levels of endotoxin (>10 EU/µg) have been shown to negatively impact transfection, protein expression and viability in standard cell lines. Plasmid DNA is considered to be endotoxin-free if it has <0.1 EU/μg.
Applications requiring endotoxin-free DNA
Endotoxin-free plasmid DNA is essential for some sensitive applications such as transfection of primary cells and research on gene therapy or plasmid vaccines. It has been demonstrated that endotoxins reduce cell viability and transfection efficiencies among different eukaryotic cell lines, such as COS-7 and Jurkat. Certain cell lines such as HuH-7, appear to be more sensitive to endotoxins.
Endotoxin removal from plasmid DNA
The difficulties posed for downstream applications by endotoxin contaminated DNA can be overcome by use of an efficient, high quality endotoxin removal kit, such as that supplied by Norgen Biotek.
Endotoxin removal (depyrogenation) methods commonly used are:
- Size exclusion methods such as ultrafiltration
- The use of affinity or ion-exchange chromatography to bind the plasmid DNA and separate it from the endotoxins. In one method, endotoxins are bound and DNA eluted.
Endotoxin removal kit from Norgen Biotek
Norgen Biotek offers a very convenient spin column format kit for endotoxin removal from DNA. Purification is based on spin column chromatography technology using their proprietary resin as a separation matrix. The resin binds DNA while endotoxins, salts and other contaminants are washed away (Figure 1).
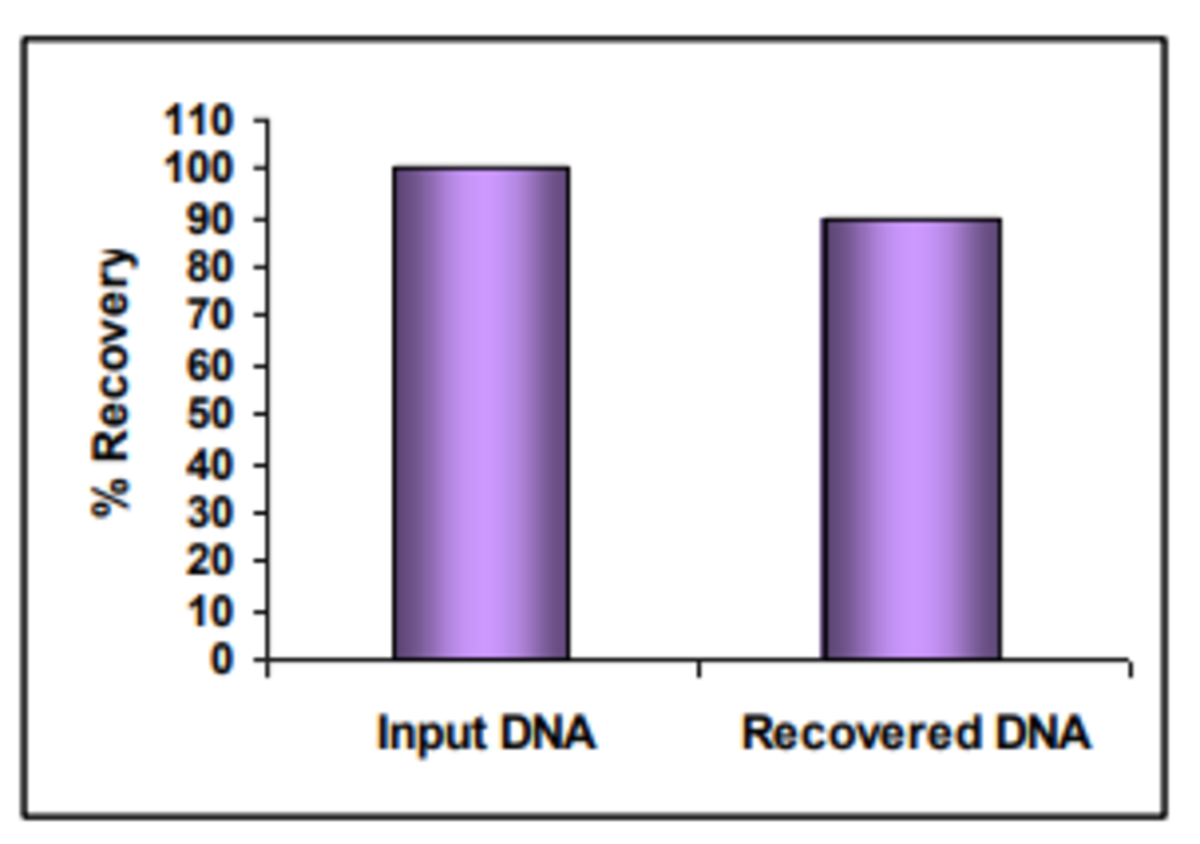
Average DNA recovery is >90% of the input DNA (Figure 2) and multiple samples can be processed in 30 minutes. The resulting plasmid DNA is effectively endotoxin free and is immediately ready for downstream applications including sequencing, cloning and transfection.
Kit specifications
| Cat-No. | Kit | Max. DNA input | Final Endotoxin levels | Average recovery | Preparation time |
|---|---|---|---|---|---|
| 22700 | Endotoxin Removal Kit (Mini) – For DNA | Up to 25 ug | ≤0.1 EU/ug DNA | > 90% | 20 minutes |
| 52200 | Endotoxin Removal Kit (Midi) – For DNA | Up to 200 ug | ≤0.1 EU/ug DNA | >90% (~80% in First Elution) | 30 minutes |
| 21900 | Endotoxin Removal Kit (Maxi) – For DNA | Up to 1 mg | ≤0.1 EU/ug DNA | > 90% | 30 minutes |
