Cytokines and growth factors are small, secreted proteins or peptides. They bind specific cell surface receptors triggering intracellular signalling pathways. This leads to upregulation or downregulation of target genes. Their signalling action can be within the same cell (autocrine), on neighbouring cells (paracrine) or on distant cells via the bloodstream (endocrine). These cell signalling molecules assist in the regulation of many biological processes including cell proliferation, differentiation and migration, metabolism, wound healing, inflammation, and angiogenesis.

Their key role in the proliferation and differentiation of living cells means cytokines and growth factors are a requirement for the maintenance of living cells cultured in a laboratory environment. Used to induce stem cell differentiation, as growth supplements in culture medium, and in various assays performed on living cells, they are essential research reagents. However, with the advent of cell and gene therapies requiring the expansion and maintenance of living cells; a source of cytokines and growth factors suitable for ex vivo expansion, maintenance and differentiation of cells is crucial. For example, Chimeric Antigen Receptor (CAR) T cell therapy is a promising new approach in the treatment of cancers. During the procedure, patient T cells are removed, cultured, and then reintroduced after genetic manipulation. The reintroduced cells express a synthetic receptor capable of detecting antigen expressed on the surface of cancerous cells, leading to their destruction. Where cells modified in the laboratory are used as a treatment for patients, the quality of raw materials such as cytokines and growth factors used in this process are of vital importance. With more of these types of therapies in development, the ease of progression from preclinical research and development to clinical trials is an important consideration for those scientists requiring cell therapy raw materials.
RUO and GMP grade cytokines and growth factors from Proteintech®
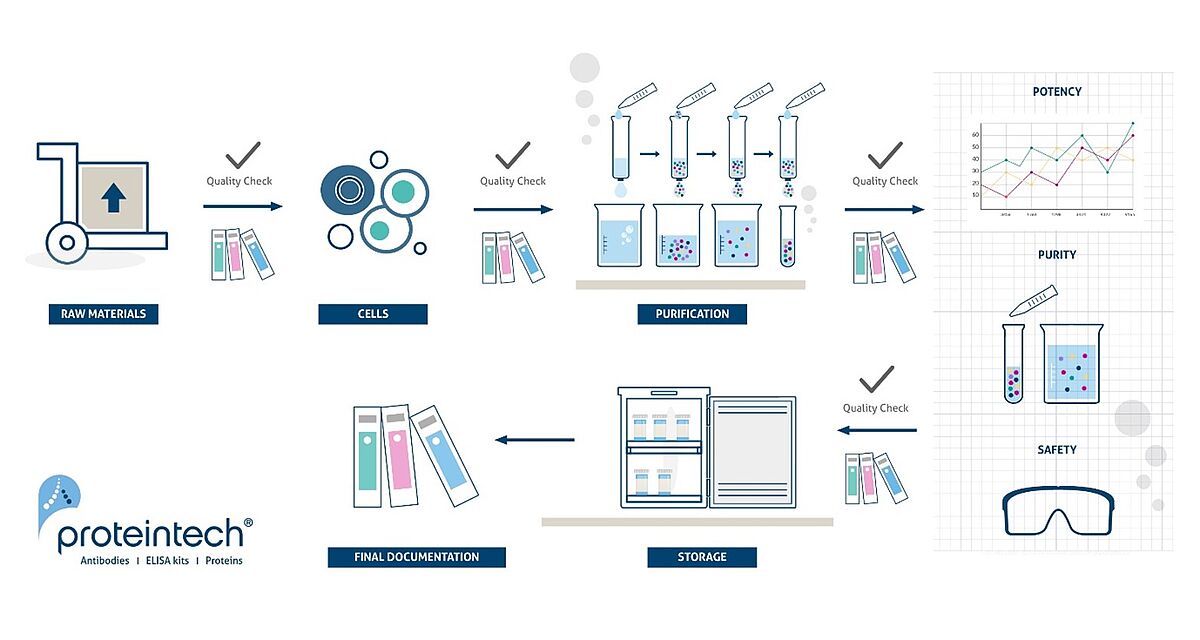
Proteintech’s research use only (RUO) and HumanKine® GMP (Good Manufacturing Practice) grade cytokines and growth factors are manufactured using the same HEK293 human cell-based expression system within a GMP facility. This guarantees that there is no variability in quality or performance between those two grades of products. The RUO proteins used for preliminary research and development can thus easily be substituted for GMP grade once a move to clinical use is planned. The GMP grade cytokines and growth factors are distinguishable from the RUO grade by certification to ISO 13485 and GMP compliance which involves a meticulous quality process.
Proteintech GMP policy and ISO 13485 quality management system ensures:
• Raw material qualification
• Regular check and qualification of clean rooms and instruments
• Validated process and standard operating procedures
• Well defined standards and In Process Quality Check (IPQC)
• Systematic and organized training program for personnel
• Documentation and traceability
• Deviations and Corrective Action Preventive Action (CAPA)

Proteintech GMP grade cytokines and growth factors for ex vivo cell manufacturing
For use as ancillary materials in clinical trials and commercial ex vivo manufacturing processes, as defined in: USP <1043>—Ancillary Materials for Cell, Gene and Tissue-Engineered Products. These highly active, recombinant GMP products are expressed in a human cell line to produce stable proteins with native folding, glycosylation, and processing. For product safety and consistency, manufacture is animal component and xenobiotic free, products derived from or by an animal are not used at any point during production. To ensure authentic structure and biological activity, they are also tag-free. As a result of these measures, HumanKine® GMP-grade proteins offer safety, purity, stability, and potency for use in ex vivo manufacturing processes.
Benefits
Benefits of animal component free and xeno free proteins:
• The final product does not contain any constituent that is either an animal tissue, body fluid or components derived from it.
• All materials from procurement to final products are stored and handled in a dedicated animal free facility.
• Final product and the process does not involve the use of materials from non- human animal sources or recombinant materials made from non-human animal sources.
Benefits of tag free proteins:
• No changes to the structure of the protein of interest.
• No interfere with the active site of the protein so therefore no alteration in biological activity.
• Tag-free recombinant proteins are more desirable for in vivo applications as they do not demonstrate the increased immunogenicity often observed in tagged proteins.
Key products
| Cat-No. | Item | Size | CHF |
|---|---|---|---|
| HZ-1138-GMP-1EACH | HumanKine® recombinant human Activin A protein- GMP grade | 1 each | Price on request |
| HZ-1045-GMP-1EACH | HumanKine® recombinant human BMP-4 protein- GMP grade | 1 each | Price on request |
| HZ-1151-GMP-1EACH | HumanKine® recombinant human FLT3 Ligand protein- GMP grade | 1 each | Price on request |